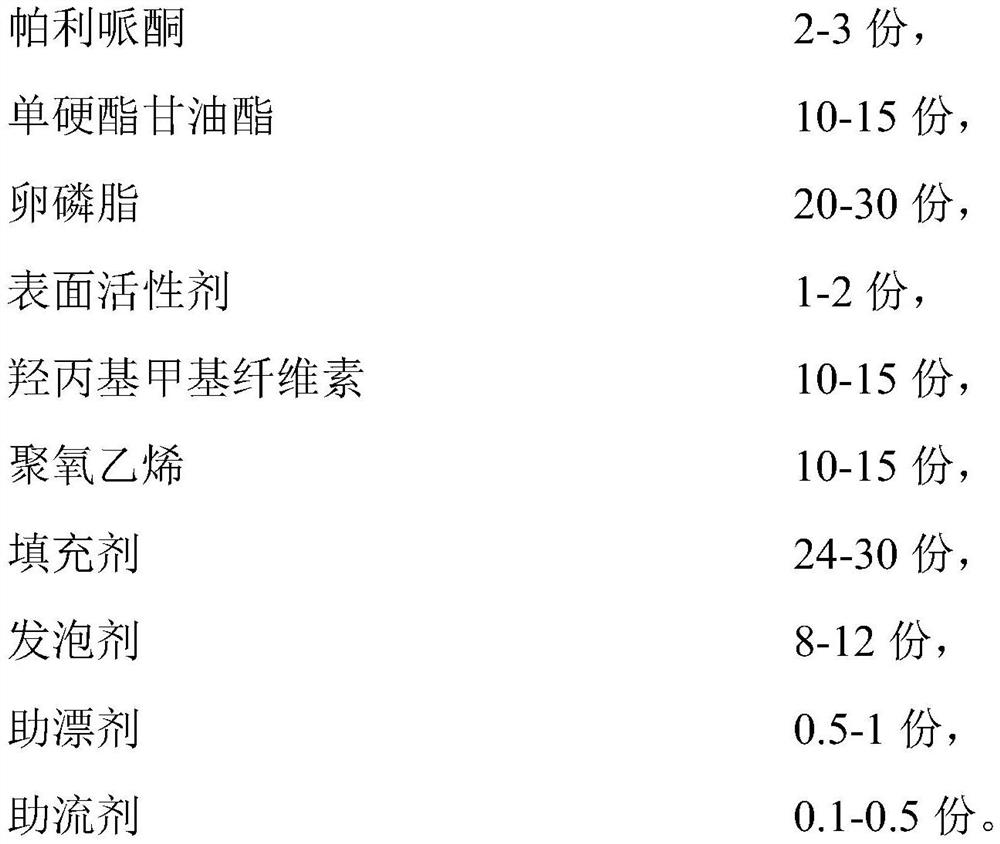
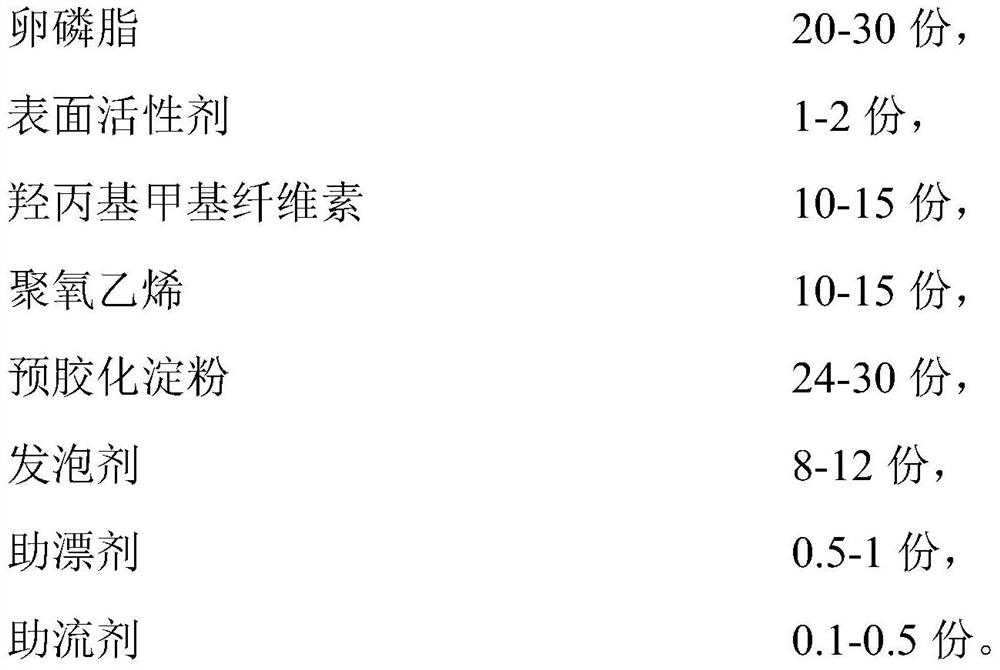
Paliperidone gastric retention tablet and preparation method thereof
A technology for paliperidone and gastric retention, which is applied in the field of medicine and can solve problems such as high difficulty, complicated process, and need to improve the sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The second aspect of the embodiment of the present application provides a preparation method of paliperidone gastric retention tablets, comprising the following steps:
[0040] S10. Mixing and dissolving the prescribed amount of glyceryl monostearate, lecithin, paliperidone and absolute ethanol to obtain an organic phase;
[0041] S20. After mixing the surfactant with the organic phase, heat and concentrate to obtain paliperidone-solid lipid nanoparticles;
[0042] S30. After mixing the paliperidone-solid lipid nanoparticles with the formulated amount of hydroxypropyl methylcellulose, polyoxyethylene, pregelatinized starch, foaming agent, bleaching aid and flow aid, dry method Tablets are obtained to obtain Paliperidone Gastric Retentive Tablets.
[0043] The preparation method of paliperidone gastric retention tablet provided by the second aspect of the present application, first, mix and dissolve the monostearyl glyceride, lecithin, paliperidone and absolute ethanol ...
Embodiment 1
[0049] Paliperidone-lipid nanoparticles, the preparation of which includes the steps of: mixing a formula amount of glyceryl monostearate, lecithin, paliperidone and absolute ethanol, and maintaining the heating temperature at 40-50°C for dissolution to obtain an organic phase; at a stirring speed of 900-1100r / min, mix and stir the surfactant and the organic phase for 1h, then heat and concentrate, and evaporate the solvent to obtain a translucent nanoemulsion, that is, paliperidone-solid lipid nano Granular colloidal solution, stored at low temperature at 0-4°C, paliperidone-solid lipid nanoparticles can be obtained; pulverized, passed through 80-mesh sieve.
[0050] Using single factor investigation test, using encapsulation efficiency and drug loading as evaluation index, investigating different factors such as paliperidone, monostearyl glyceride, surfactant type, surfactant concentration, etc., to prepare paliperidone- Effect of Lipid Nanoparticles, Again Using Orthogonal ...
Embodiment 2
[0065] Paliperidone gastric retention tablet, its preparation comprises steps:
[0066] ①Mix 21g of glyceryl monostearate, 41g of lecithin, 3g of paliperidone and 2g of absolute ethanol in the prescribed amount, and dissolve at a heating temperature of 40-50°C to obtain an organic phase; stir at 900-1100r / min Mix and stir 2g of 0.4% poloxamer aqueous solution F68 (m / V) aqueous solution with the organic phase for 1 hour, then heat and concentrate, and evaporate the solvent to obtain a translucent nanoemulsion, that is, paliperidone-solid lipid Quality nanoparticle colloid solution, stored at 0-4°C at low temperature, paliperidone-solid lipid nanoparticles can be obtained; pulverize, pass through 80-mesh sieve.
[0067] ②Weigh the prescription amount of HPMC-K15M, polyoxyethylene, pregelatinized starch, sodium bicarbonate, glyceryl monostearate, magnesium stearate, pass through an 80 mesh sieve, and use the paliperidone-solid obtained in step ① The lipid nanoparticles are evenl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com