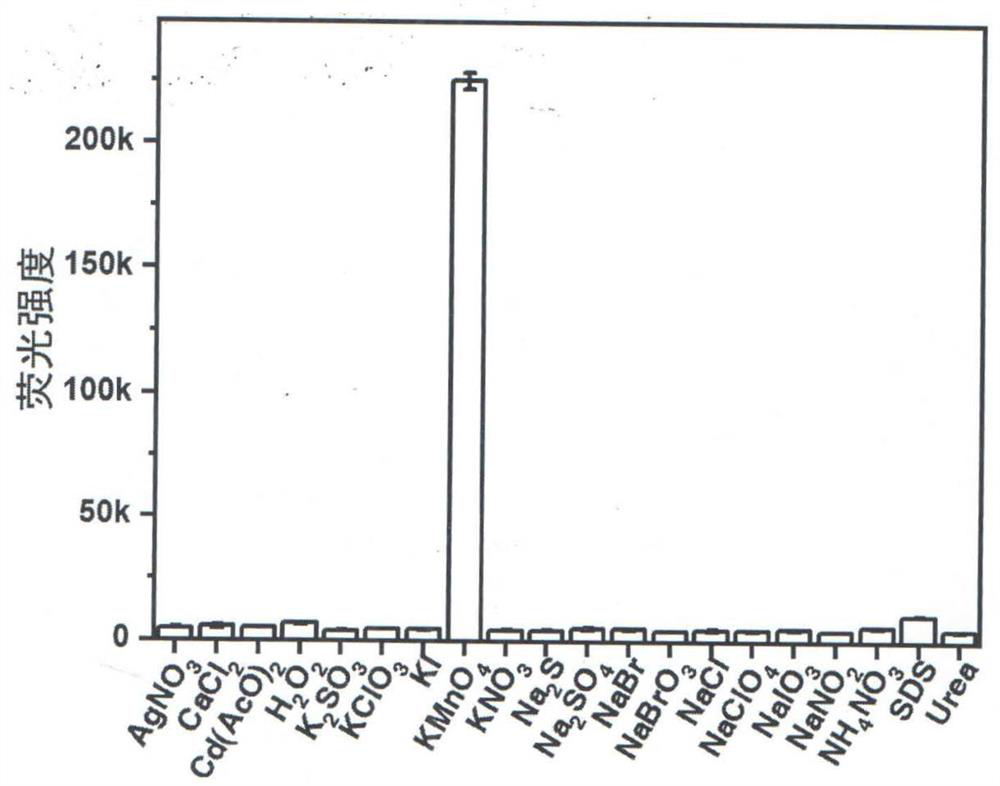
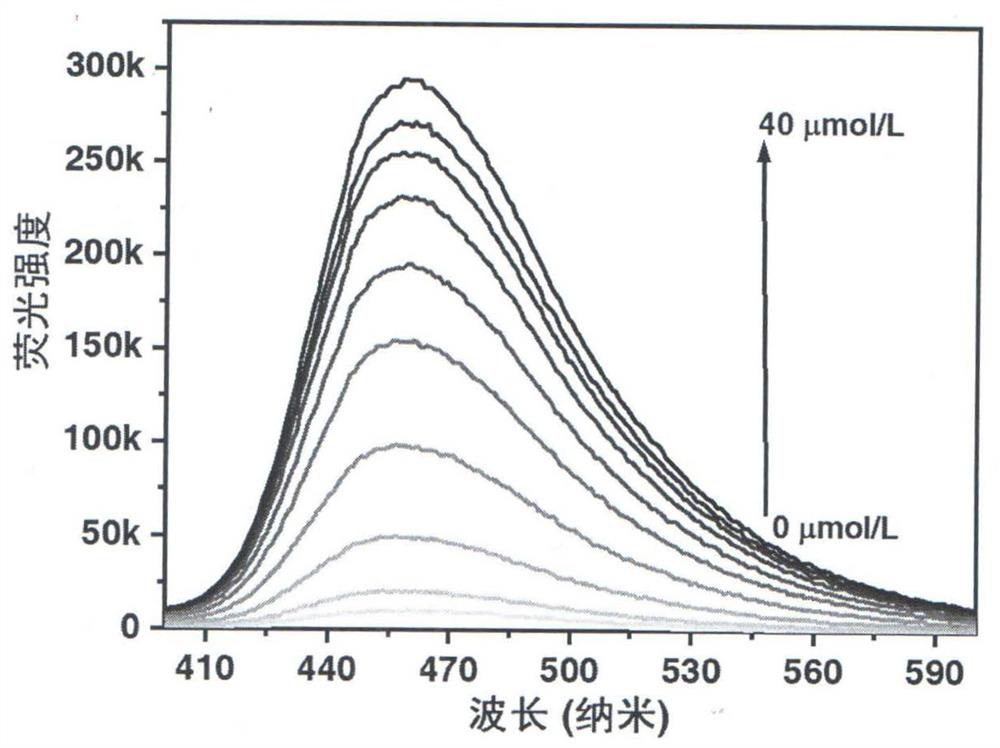
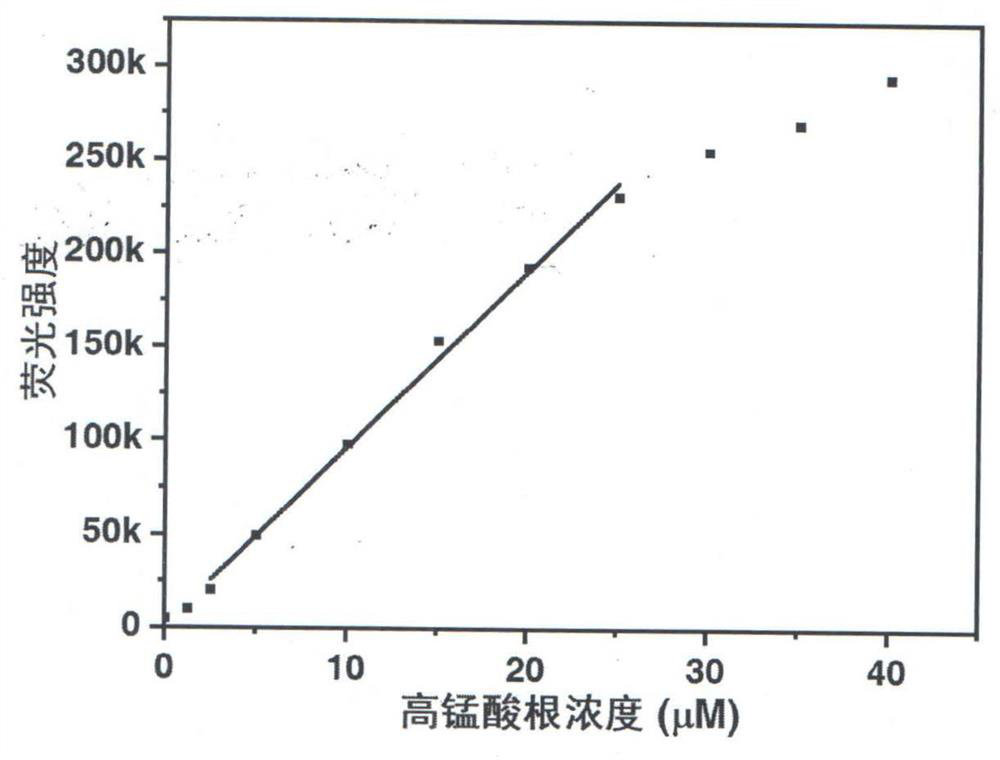
A kind of method for detecting permanganate with coumarin-based probe
A permanganate and coumarin-based technology is applied in the field of environmental and non-standard explosive detection, which can solve the problems of time delay of oxidation reaction, low molar absorption coefficient, low quantum yield, etc., and achieves simple production, obvious signal, Sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of probe molecule 2-oxo-2hydro-benzopyran-7-acrylate:
[0032] a. Dissolve 7-hydroxybenzopyran-2-one (5mmol, 0.81g) in 50mL of tetrahydrofuran, add it to a 200mL three-necked round-bottomed flask, add organic base pyridine (5mmol, 0.402mL) and stir, pass through Under nitrogen protection, ice bath to 0°C, slowly add acryloyl chloride (5 mmol, 0.398 mL) dropwise for 2 h, warm to room temperature for 1 h, and stop the reaction;
[0033] b, wash with deionized water to neutrality after the reaction is finished, then extract water with dichloromethane, wash with sodium chloride, dry with anhydrous magnesium sulfate, suction filtration, spin dry to obtain a crude product;
[0034] c. The obtained crude product is subjected to gradient elution with a forward silica gel chromatographic column, the eluent is ethyl acetate / petroleum ether, and the eluent ratio is gradually increased from 0:7 to 2:7, and a white color is obtained after separation and purification. The ...
Embodiment 2
[0036] Synthesis of probe molecule 2-oxo-2hydro-benzopyran-7-acrylate:
[0037] a. Dissolve 7-hydroxybenzopyran-2-one (5mmol, 0.81g) in 50mL of chloroform, add it to a 200mL three-necked round-bottomed flask, and then add organic base triethylamine (25.5mmol, 3.55mL ) and stir. Pass nitrogen protection, ice bath to 0 °C, slowly add acryloyl chloride (25 mmol, 1.99 mL) dropwise for 2 h, warm to room temperature for 24 h, and stop the reaction;
[0038] b, wash with deionized water to neutrality after the reaction is finished, then extract water with dichloromethane, wash with sodium chloride, dry with anhydrous magnesium sulfate, suction filtration, spin dry to obtain a crude product;
[0039] c. The obtained crude product is subjected to gradient elution with a forward silica gel chromatographic column, the eluent is ethyl acetate / petroleum ether, and the eluent ratio is gradually increased from 0:7 to 2:7, and a white color is obtained after separation and purification. The s...
Embodiment 3
[0041] Synthesis of probe molecule 2-oxo-2hydro-benzopyran-7-methacrylate:
[0042] a. Dissolve 7-hydroxybenzopyran-2-one (5mmol, 0.81g) in 50mL of tetrahydrofuran, add it to a 200mL three-necked round-bottomed flask, add organic base pyridine (5mmol, 0.402mL) and stir, pass through Under nitrogen protection, ice bath to 0°C, slowly add methacryloyl chloride (5 mmol, 0.484 mL) dropwise for 2 h, warm to room temperature for 1 h, and stop the reaction;
[0043] b, wash with deionized water to neutrality after the reaction is finished, then extract water with dichloromethane, wash with sodium chloride, dry with anhydrous magnesium sulfate, suction filtration, spin dry to obtain a crude product;
[0044] c. The obtained crude product is subjected to gradient elution with a forward silica gel chromatographic column, the eluent is ethyl acetate / petroleum ether, and the eluent ratio is gradually increased from 0:7 to 2:7, and a white color is obtained after separation and purificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com