Polymer electrolyte and preparation method and application thereof
A polymer and electrolyte technology, applied in non-aqueous electrolytes, solid electrolytes, non-aqueous electrolyte batteries, etc., can solve the problem that it is difficult to reduce the risk of thermal runaway of high-energy lithium batteries, polymer electrolytes do not have secondary cross-linking, and cannot meet high ratio It can quickly reduce battery temperature, avoid battery thermal runaway, and prevent battery thermal runaway.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The raw material ratio used to prepare the polymer electrolyte is shown in Table 1, and the LiTFSI / EMC solution was prepared in a glove box filled with argon. Will Add acrylonitrile and acrylonitrile to the above solution, and add the initiator AIBN. After it is completely dissolved, inject the solution into a lithium-ion battery containing positive and negative electrode materials, and place it at 60°C for in-situ polymerization. After 8 hours, the required polymer electrolyte battery.
[0065] Table 1:
[0066]
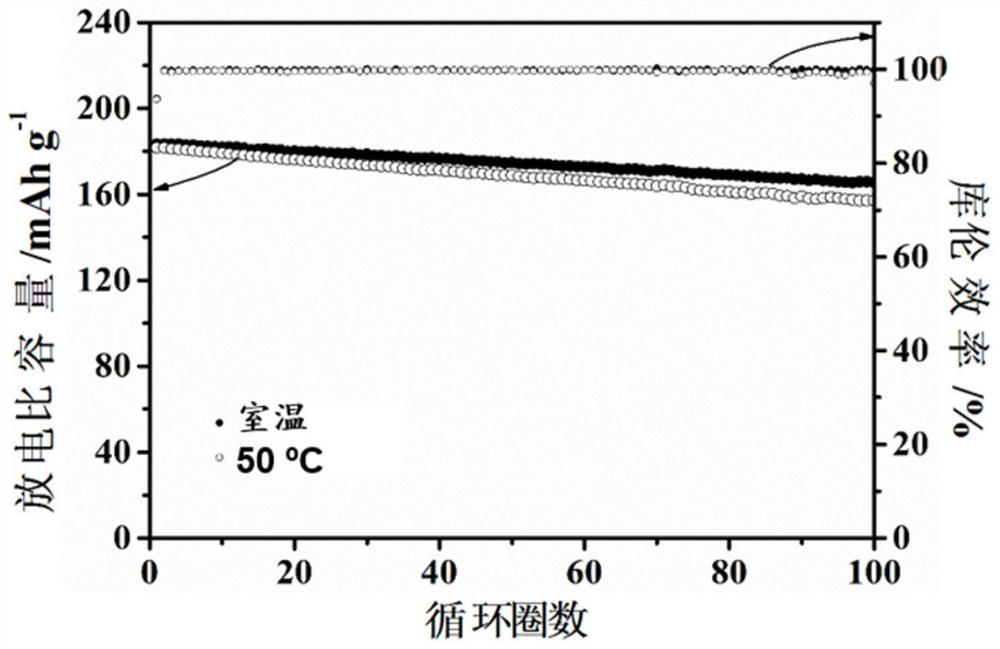
[0067] The electrolyte obtained from the above Example 1 has high ionic conductivity, wide electrochemical window and high tensile strength (Table 1). The NCM622 / Li metal full battery was assembled with the electrolyte obtained above, and the capacity retention rates were 90% and 87% after cycling at room temperature and 50°C for 100 cycles at operating voltages of 2.5-4.4V and 0.1C, respectively (such as figure 1 Shown), it can be seen that the obtain...
Embodiment 2
[0070] The ratio of raw materials used to prepare the polymer electrolyte is shown in Table 2. The urethane acrylate prepared in advance according to the monomer ratio shown in Table 2 was dissolved in NMP, mixed uniformly, scraped and coated on PET, and dried to obtain a polymer film. After the polymer film was punched, the Fully swell in the solution to obtain a polymer electrolyte membrane. The electrolyte membrane is combined with corresponding positive and negative electrode materials to assemble a lithium battery.
[0071] Table 2:
[0072]
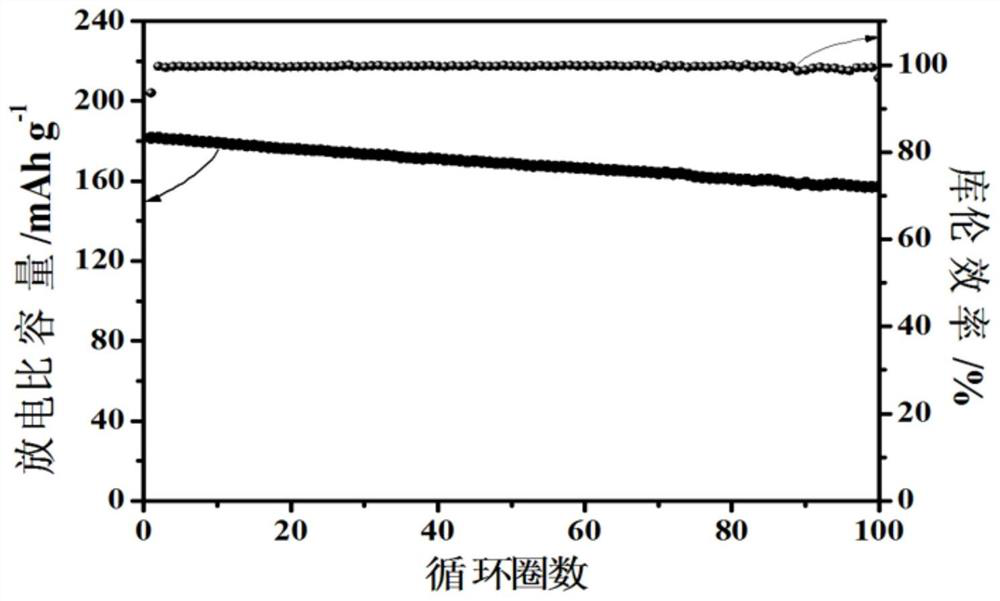
[0073] The electrolyte obtained from the above Example 2 has high ionic conductivity, wide electrochemical window and high tensile strength (Table 2). The above-mentioned electrolytes were assembled into NCM811 / lithium metal full batteries, and the capacity retention rate was 89% after 100 cycles at an operating voltage of 2.5-4.3V and 2.0C (such as image 3 Shown), it can be seen that the obtained polymer electrolyte has ex...
Embodiment 3
[0076] The ratio of raw materials used to prepare the polymer electrolyte is shown in Table 3. In a glove box filled with argon, the Vinylene carbonate and LiDFOB were mixed together to make a solution, and the initiator BPO was added. After it was completely dissolved, the solution was injected into a lithium-ion battery containing positive and negative materials, and placed at 80°C for in-situ polymerization. After 6 hours Obtain the required polymer electrolyte battery.
[0077] table 3:
[0078]
[0079]
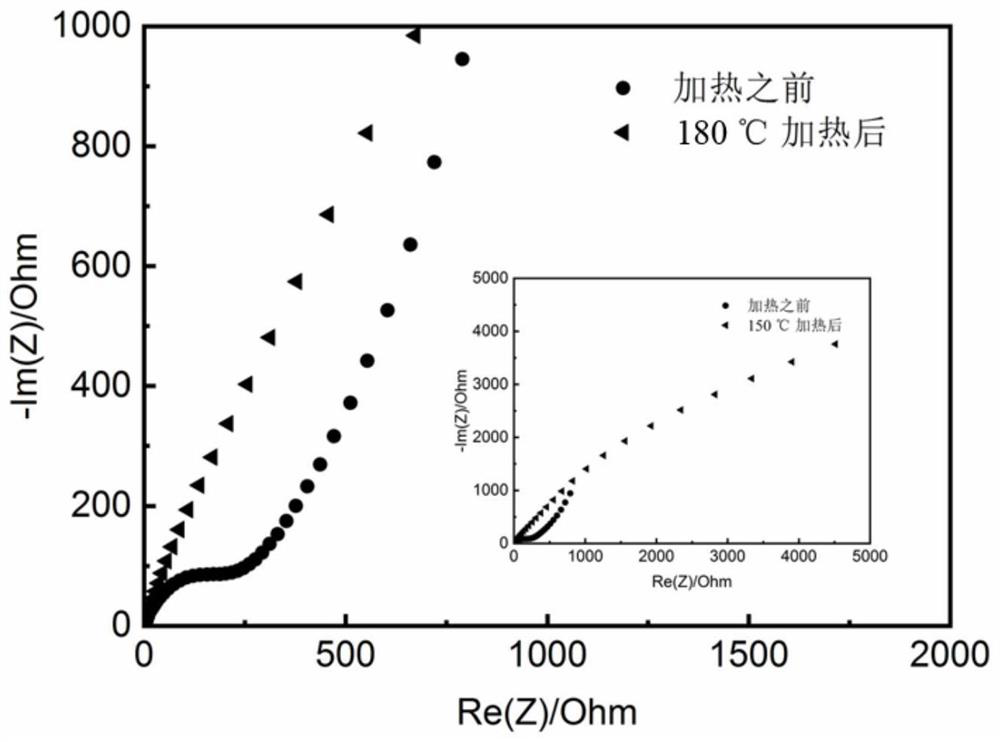
[0080] The electrolyte obtained from the above Example 3 has high ionic conductivity, wide electrochemical window and high tensile strength (Table 3). The lithium cobaltate / graphite full battery was assembled with the above electrolyte, and the capacity retention rate was 91% after 200 cycles at 50°C at an operating voltage of 2.5-4.4V and 0.5C (such as Figure 5 Shown), it can be seen that the obtained polymer electrolyte has excellent electrochemical performan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com