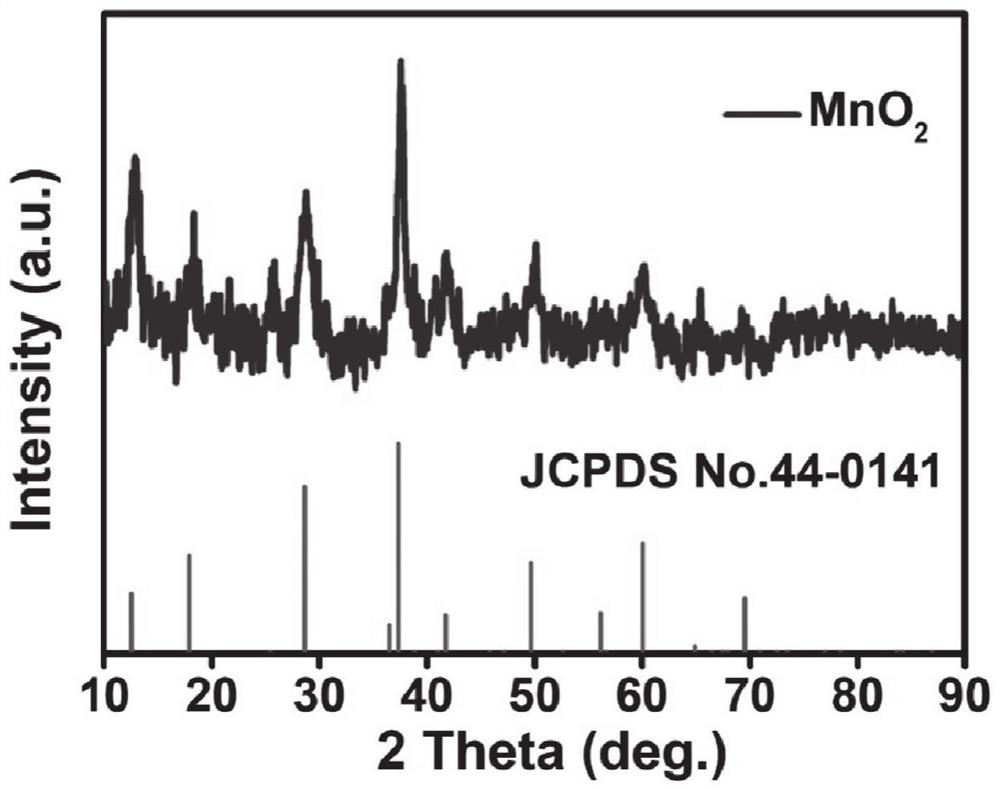
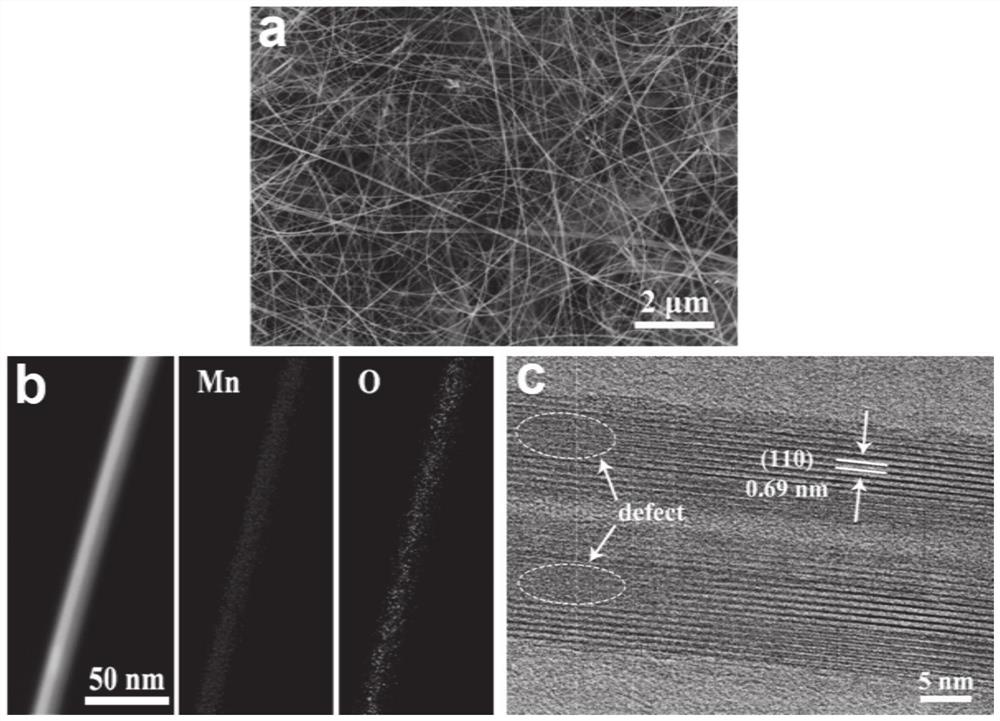
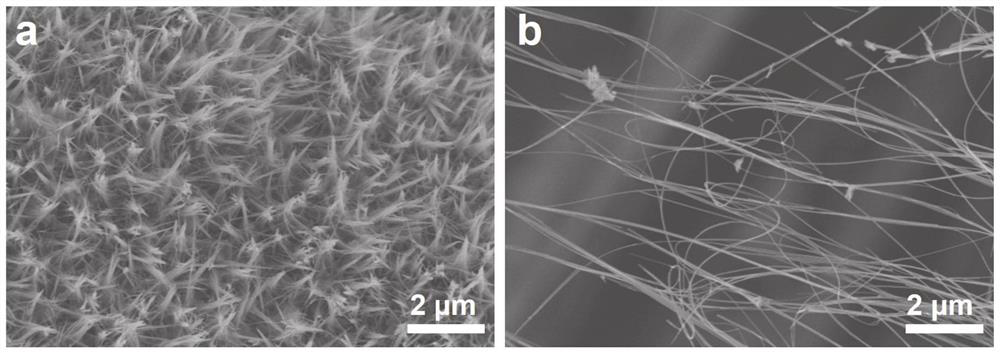
Manganese dioxide super-long nanowire catalyst with oxygen vacancies as well as preparation method and application of manganese dioxide super-long nanowire catalyst
A technology of manganese dioxide and nanowires, applied in manganese oxide/manganese hydroxide, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problems of insufficient activity of active sites and poor nitrogen adsorption capacity, etc. Achieve the effects of simple and adjustable preparation method, enhanced adsorption and activation, and enlarged contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A MnO with oxygen vacancies 2 The preparation method of ultralong nanowire catalyst, the steps are as follows:
[0039] (1) Ultrasonic cleaning of carbon paper conductive substrate:
[0040] First cut with scissors to obtain a carbon paper conductive substrate with a size of 2cm×3cm, then ultrasonically clean it with dilute hydrochloric acid, acetone, and ethanol for 20 minutes, and finally store it in an ethanol solvent.
[0041] (2) One-step hydrothermal synthesis of MnO with oxygen vacancies 2 Ultra-long nanowires:
[0042] At room temperature, 3 mmol KMnO 4 Dissolve in 40 mL deionized water and stir magnetically for 20 minutes to obtain a purple solution. Then the solution is transferred to a reaction kettle with 50 milliliters of polytetrafluoroethylene liners, and the clean carbon paper obtained in (1) is placed in the kettle, subjected to hydrothermal reaction at 220° C. for 36 hours, and cooled naturally to obtain Uniform growth of MnO on paper 2 ultra-lon...
Embodiment 2
[0051] A MnO with oxygen vacancies 2 The preparation method of ultralong nanowire catalyst, the steps are as follows:
[0052] (1) Ultrasonic cleaning of carbon paper conductive substrate:
[0053] First cut with scissors to obtain a carbon paper conductive substrate with a size of 2cm×3cm, then ultrasonically clean it with dilute hydrochloric acid, acetone, and ethanol for 20 minutes, and finally store it in an ethanol solvent.
[0054] (2) One-step hydrothermal synthesis of MnO with oxygen vacancies 2 Ultra-long nanowires:
[0055] At room temperature, 2 mmol KMnO 4 Dissolve in 40 mL deionized water and stir magnetically for 20 minutes to obtain a purple solution. Then the solution is transferred to a reaction kettle with 50 milliliters of polytetrafluoroethylene liners, and the clean carbon paper obtained in (1) is placed in the kettle, subjected to hydrothermal reaction at 220° C. for 36 hours, and cooled naturally to obtain Uniform growth of MnO on paper 2 ultra-lon...
Embodiment 3
[0056] Embodiment 3 electrocatalytic nitrogen reduction experiment
[0057] 1. Test method:
[0058] The electrocatalytic nitrogen reduction ammonia production test was performed using a three-electrode H-type electrolytic cell device and recorded by an electrochemical workstation (CHI 750E). The electrolytic cell is separated by a Nafion membrane from the anode compartment and the cathode compartment. MnO with oxygen vacancies to be grown on carbon paper 2 The ultra-long nanowire (the product prepared in Example 1) was cut to 1 cm×1 cm as the working electrode, the carbon rod as the counter electrode, and the Ag / AgCl electrode as the reference electrode. The test was carried out at room temperature with 0.1mol / L sodium sulfate solution as the electrolyte.
[0059] 2. Electrocatalytic nitrogen reduction activity test:
[0060] MnO with oxygen vacancies grown on carbon paper 2 The ultra-long nanowire is used as the working electrode, and the cyclic voltammetry test is perf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com