Composite bifidobacterium preparation capable of resisting allergy, enhancing immunity, reducing blood sugar, reducing fat and losing weight and preparation method of composite bifidobacterium preparation
A compound bifidobacteria, lipid-lowering and weight loss technology, applied in the directions of bifidobacteria, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low concentration of viable bacteria, poor fermentation effect, low bacterial activity, etc. To achieve the effect of increasing the number of bacteria, increasing vitality, and reducing drying stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation method includes the following steps:
[0059] (1) Mix Bifidobacterium bifidum Miuyo-01, Bifidobacterium infantis Miuyo-21, Bifidobacterium breve Miuyo-31 and Bifidobacterium lactis Miuyo-11 according to the ratio to form a compound bifidobacterium, and put it into the liquid The first anaerobic culture is carried out in the culture medium, and the culture solution produced is centrifuged to obtain the wet cells of bifidobacteria, which are set aside;
[0060] (2) Mix the wet thalline of the compound bifidobacterium obtained in step (1) with the solid medium, carry out anaerobic cultivation for the second time in a sealed state, carry out low-temperature drying after the cultivation is completed, and form a solid compound bifidobacterium ;
[0061] (3) The composite bifidobacteria solids obtained in step (2) are pulverized, anaerobically granulated, and anaerobically coated to obtain the composite bifidobacterium preparations for anti-allergy, increased ...
Embodiment 1
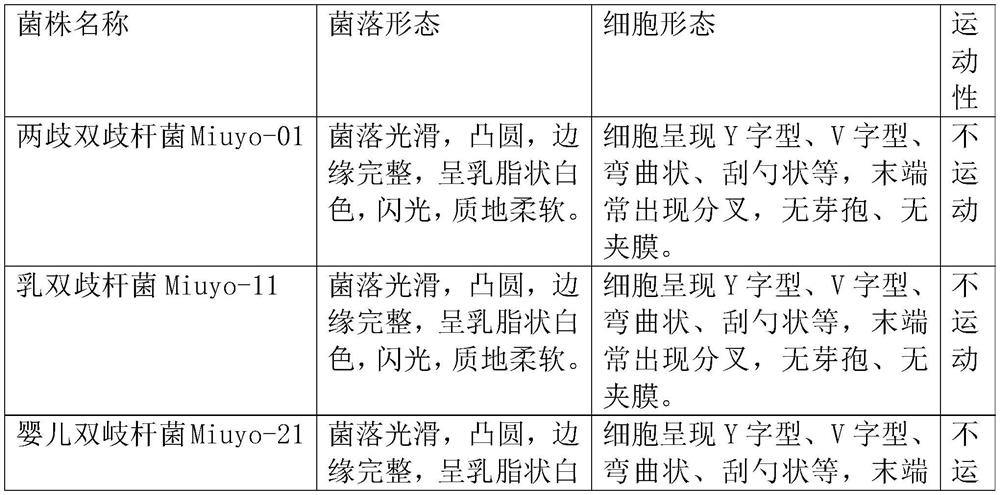
[0065] Isolation, strain characteristics and identification of embodiment 1 bacterial strain
[0066] 1.1 Sample collection:
[0067] Select healthy infants who were 3 months to 1 year after birth and have not used antibacterial drugs recently, and collect samples from their feces for isolation, that is, the isolated strains come from the intestinal tract of healthy infants.
[0068] 1.2 Isolation of strains
[0069] Take 5-10g of infant feces samples, put them in a sterile 50ml centrifuge tube, add 20ml of sterile normal saline, shake and mix well, put them in an anaerobic incubator at 37°C, let them stand for overnight culture, draw 1ml of the sample solution, and Sterile normal saline was sequentially diluted 10 times to 10 -1 , 10 -2 , 10 -3 , 10 -4 , 10 -5 , 10 -6 For each gradient, 100 μl of bacterial suspension was coated on the improved MRS plate, and placed in an anaerobic incubator at 37°C for upside-down culture. After 24 hours, colonies were selected from th...
Embodiment 2
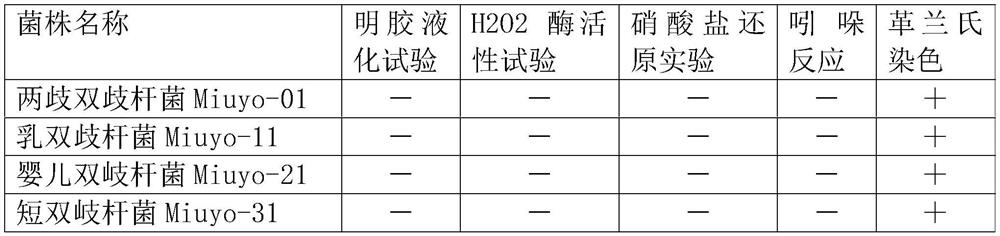
[0090] The characteristic of embodiment 2 bacterial strains
[0091] 2.1 Determination of the number of live bacteria in the product
[0092] After gradient dilution with sterilized physiological saline to a certain multiple, adopt the improved MRS agar medium plate pouring method, and count the total number of colonies after culturing at 37°C for 48 hours.
[0093] 2.2 Growth at different pH
[0094] Inoculate the above-mentioned 5 activated strains in the improved MRS liquid medium, obtain the seed liquid of the strain after cultivation, take 1 mL of the seed liquid and inoculate in 19 mL of the improved MRS liquid medium with pHs of 6.0, 6.5, 7.0 and 7.5 respectively , anaerobic culture at 37°C for 24h, measure the initial and end OD600 values (that is, the absorbance value at 600nm wavelength, which can usually be used to compare the cell density or growth of the bacteria in the culture solution), and the pH7 The OD600 value of 0.0 is used as a contrast (that is, the num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com