A long-acting recombinant human filaggrin expressed by Saccharomyces cerevisiae and its application
A technology of Saccharomyces cerevisiae and filaggrin, applied in the emerging biomedical field, can solve the problems of low biological activity of human filaggrin, easy formation of inclusion bodies, and restrictions on popularization and application, and achieve low cost, reduced production cost, and improved production process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
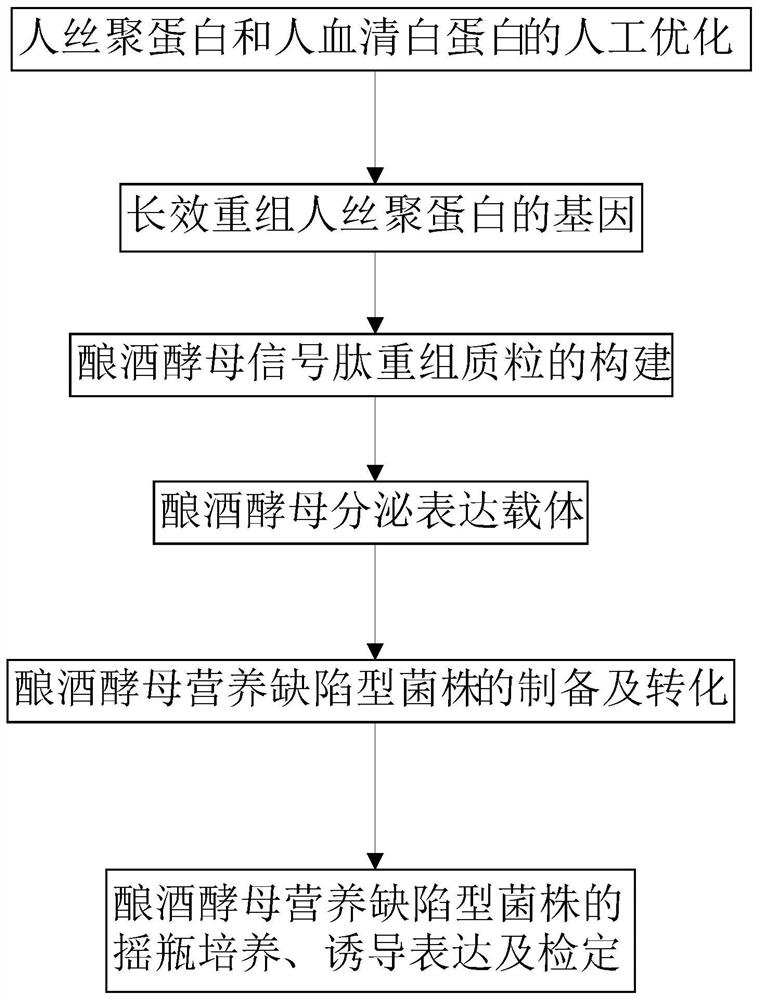
Image
Examples
Embodiment 1
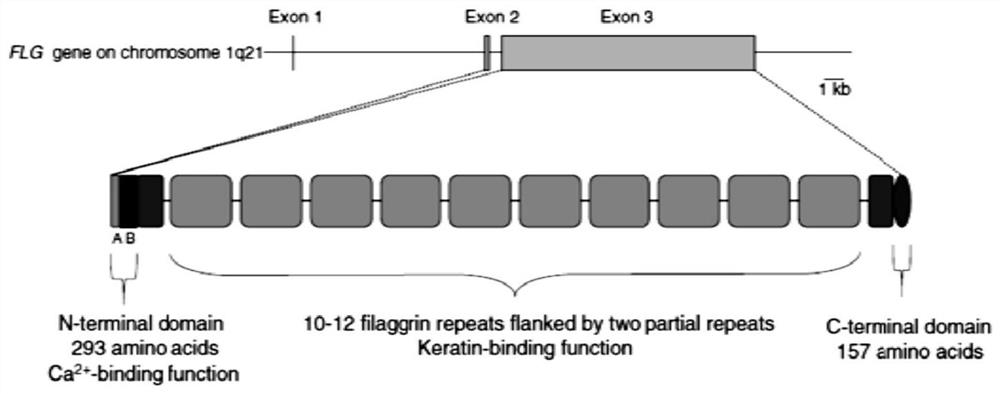
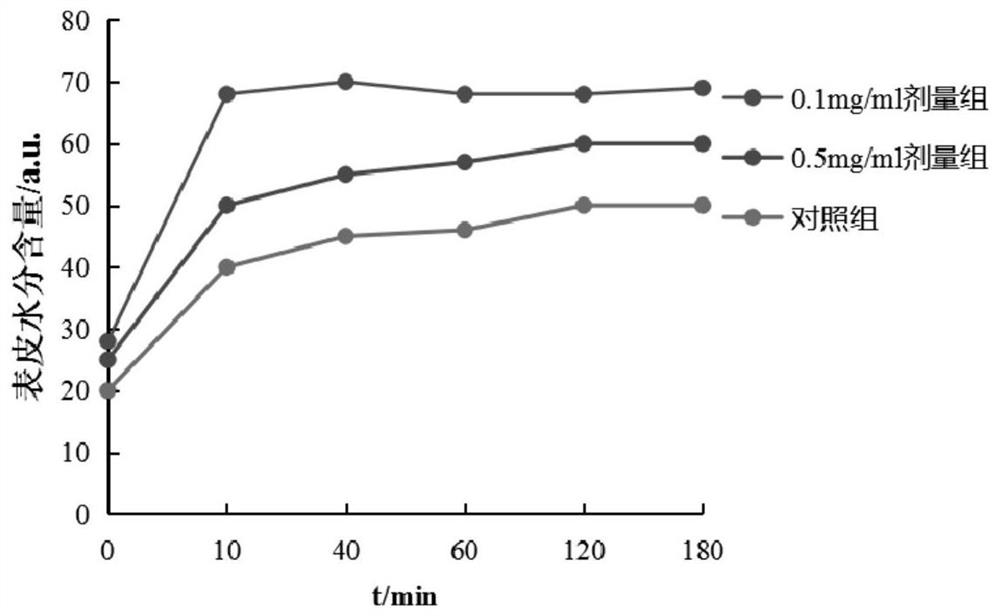
[0039] This example discloses a long-acting recombinant human filaggrin expressed by Saccharomyces cerevisiae. The long-acting recombinant human filaggrin is a recombinant protein formed by fusing a monomer structure at the C-terminal of the natural human FLG repeat unit with HSA, and the recombinant protein can prevent the loss of epidermal water. And the recombinant protein should fuse hFLG truncated protein with HSA protein, which effectively improves the stability of the recombinant protein, and has simple production process, low cost, uniform product and no immunogenicity.
[0040] The nucleotide sequence of the long-acting recombinant human filaggrin is shown in Seq ID NO.1, and the amino acid sequence corresponding to the nucleotide sequence of the long-acting recombinant human filaggrin is shown in Seq ID NO.2.
[0041] The amino acid sequence of long-acting recombinant human filaggrin includes the amino acid sequence of filaggrin and the amino acid sequence of human s...
Embodiment 2
[0052] This embodiment discloses a signal peptide recombinant plasmid of Saccharomyces cerevisiae. The Saccharomyces cerevisiae signal peptide recombinant plasmid uses the Saccharomyces cerevisiae as described in Example 1 to express the long-acting recombinant human filaggrin gene.
[0053] The construction of the Saccharomyces cerevisiae signal peptide recombinant plasmid comprises the following steps:
[0054] Firstly, synthetic primers were designed according to the sequence of Saccharomyces cerevisiae Mfα gene, and the synthetic primers included forward primer Mfα-F and reverse primer Mfα-R. In this example, the forward primer Mfα-F is shown in Seq ID NO.3, and the reverse primer Mfα-R is shown in Seq ID NO.4.
[0055] Secondly, the signal peptide Mfα gene in the yeast is amplified by RT-PCR to obtain the RT-PCR amplification product, and the RT-PCR amplification product is recovered by gel to obtain the gel recovery product.
[0056] Then the product recovered from the...
Embodiment 3
[0070] This embodiment provides a secretory expression vector of Saccharomyces cerevisiae, which contains the gene for expressing long-acting recombinant human filaggrin in Saccharomyces cerevisiae as described in Example 1. The construction of the Saccharomyces cerevisiae secretion expression vector pYES2 / CT-Mfα-hFLG-HSA specifically includes the following steps:
[0071] The plasmid pYES2 / CT-Mfα and plasmid pMD19-T-hFLG-HSA were double-digested with endonuclease BamH I and endonuclease Xba I respectively. The enzyme digestion reaction system is:
[0072]
[0073] The enzyme digestion reaction was carried out in a metal bath at 37°C for 3 hours, and the digestion products were detected by 2% agarose gel electrophoresis, and the hFLG-HSA gene fragment and the pYES2 / CT-Mfα vector were recovered by gel cutting, and then ligated with T4 DNA ligase. The connection reaction system is:
[0074]
[0075] The reaction conditions were 14 hours at 16°C, and the recombinant plasmi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com