Bimetal phosphide inlaid carbon hollow nanocage as well as preparation method and application thereof
A bimetallic and carbon-hollow technology, applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve problems such as battery performance degradation, increased polysulfide content, and difficulty in obtaining theoretical specific capacity. , to achieve the effects of increased discharge specific capacity, improved electrochemical performance, and high cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method for a lithium-sulfur battery multifunctional diaphragm, comprising the following steps:
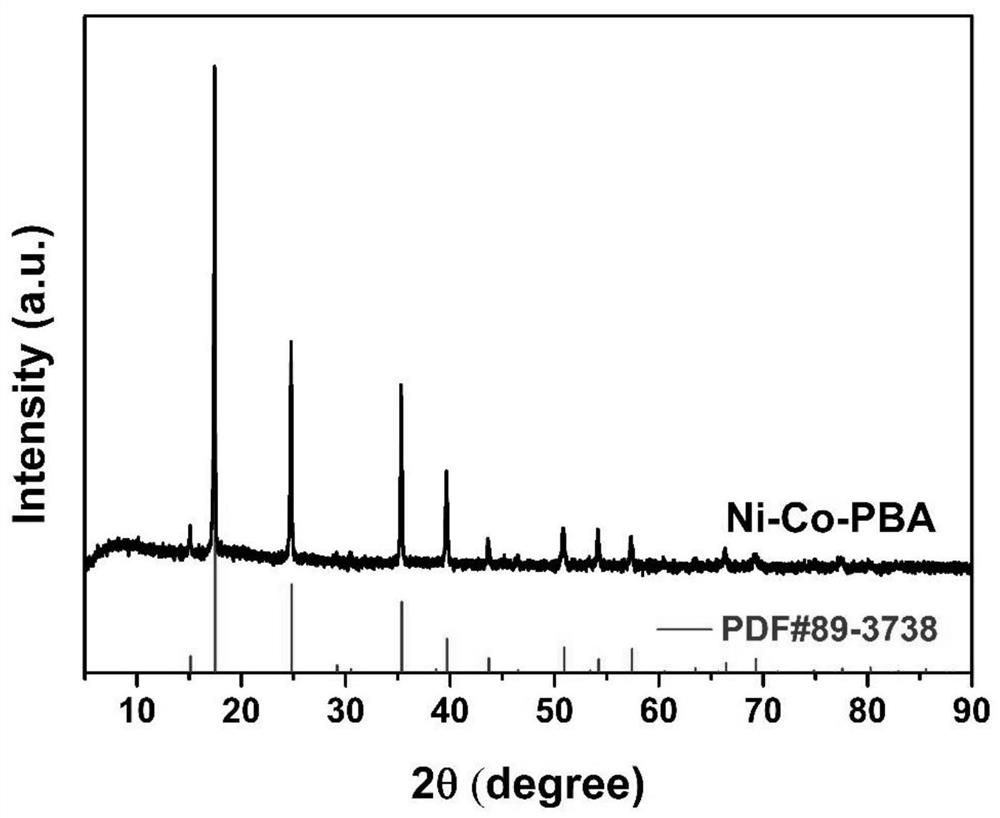
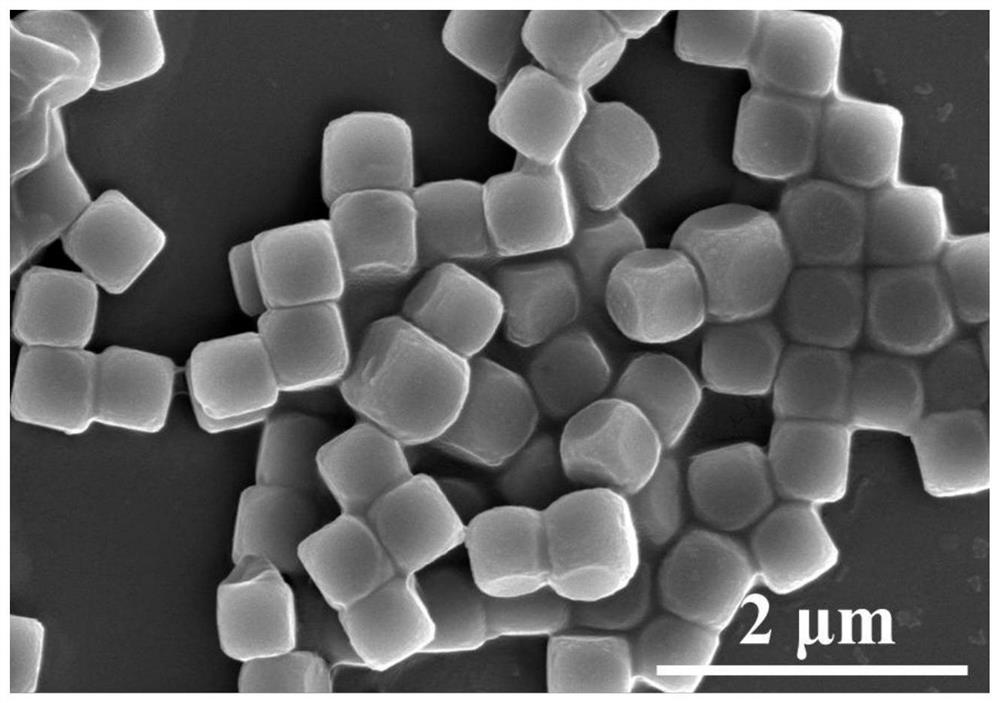
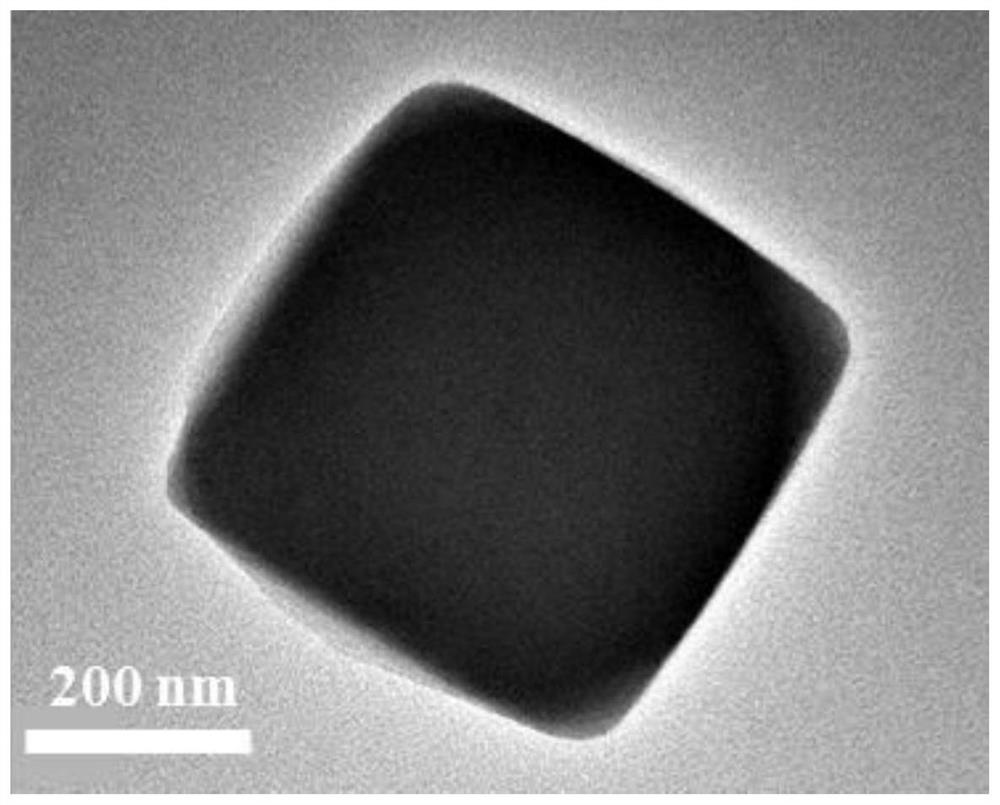
[0046] (1) Preparation of nickel-cobalt Prussian blue derivative (Ni-Co-PBA): 1.75 g (20 mmol) of nickel nitrate hexahydrate and 2.65 g (75 mmol) of trisodium citrate were dissolved in 200 ml of In deionized water, solution A was formed; 1.33 grams of potassium cobaltcyanide was dissolved in 200 ml of deionized water to form solution B; then A and B solutions were mixed, and the whole process was completed during stirring; after half an hour of stirring at room temperature , standing for 18 hours, and then washed by centrifugation to collect a light blue precipitate, which was vacuum-dried to obtain a nickel-cobalt Prussian blue derivative.
[0047] (2) Dopamine hydrochloride coated nickel-cobalt Prussian blue derivative (Ni-Co-PBA@PDA): 200 mg of prepared Ni-Co-PBA powder was dispersed in 10 mmol per liter of tris(hydroxymethyl)aminomethane In a mixed solutio...
Embodiment 2
[0052] A preparation method for a lithium-sulfur battery multifunctional diaphragm, comprising the following steps:
[0053] (1) Preparation of nickel-cobalt Prussian blue derivatives (Ni-Co-PBA): 0.35 grams of nickel nitrate hexahydrate and 0.53 grams of trisodium citrate were dissolved in 200 milliliters of deionized water to form solution A; 0.266 One gram of potassium cobaltcyanide was dissolved in 200 ml of deionized water to form solution B; then A and B solutions were mixed, and the whole process was completed during stirring; after half an hour of stirring at room temperature, it was left to stand for 18 hours, and then washed by centrifugation , a light blue precipitate was collected, and nickel-cobalt Prussian blue derivatives were obtained after vacuum drying.
[0054] (2) Dopamine hydrochloride coated nickel-cobalt Prussian blue derivative (Ni-Co-PBA@PDA): 200 mg of prepared Ni-Co-PBA powder was dispersed in 15 mmol per liter of tris(hydroxymethyl)aminomethane In ...
Embodiment 3
[0059] A preparation method for a lithium-sulfur battery multifunctional diaphragm, comprising the following steps:
[0060] (1) Preparation of nickel-cobalt Prussian blue derivative (Ni-Co-PBA): 1.75 grams of nickel nitrate hexahydrate and 2.65 grams of trisodium citrate were dissolved in 200 milliliters of deionized water to form solution A; 1.33 One gram of potassium cobaltcyanide was dissolved in 200 ml of deionized water to form solution B; then A and B solutions were mixed, and the whole process was completed during stirring; after half an hour of stirring at room temperature, it was left to stand for 18 hours, and then washed by centrifugation , a light blue precipitate was collected, and nickel-cobalt Prussian blue derivatives were obtained after vacuum drying.
[0061] (2) Nickel-cobalt Prussian blue derivative coated with dopamine hydrochloride (Ni-Co-PBA@PDA): 200 mg of prepared Ni-Co-PBA powder was dispersed in 5 mmol per liter of tris(hydroxymethyl)aminomethane I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com