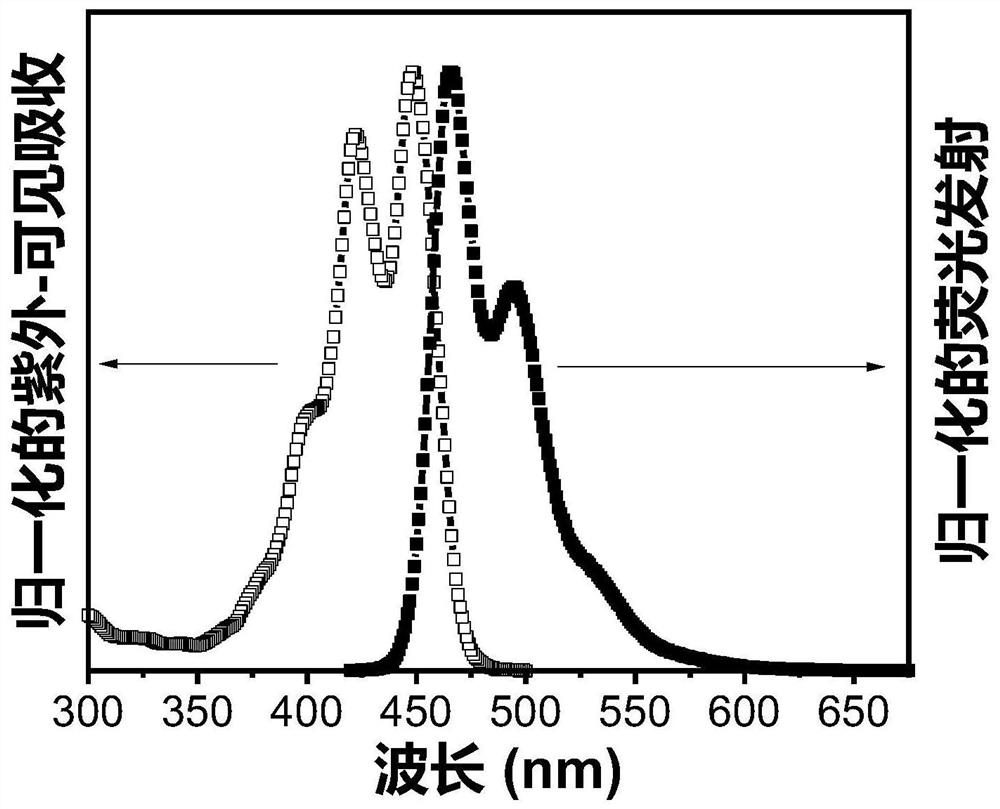
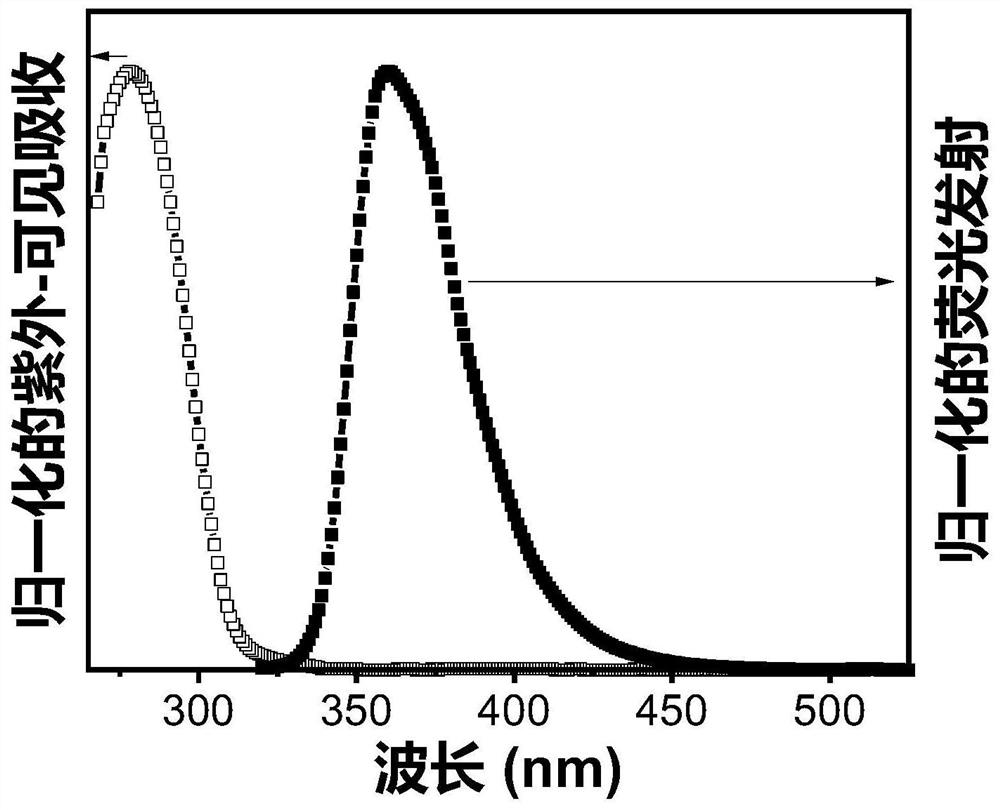
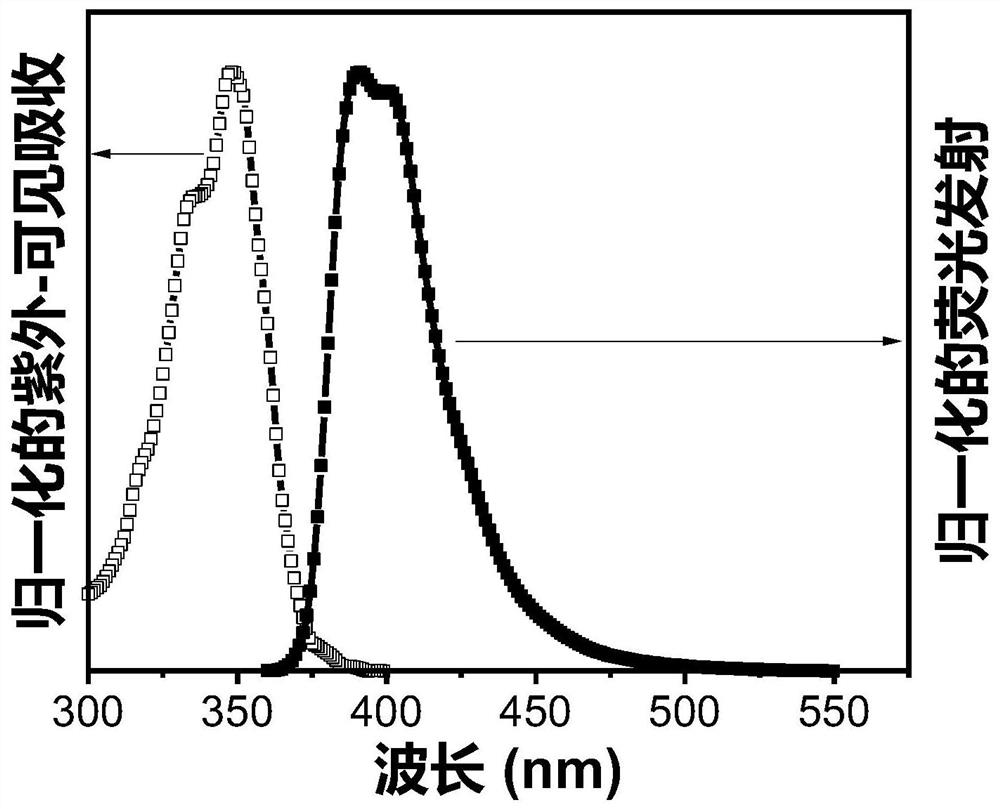
Triplet annihilation agent and preparation method and application thereof
A triplet annihilation and triplet technology, applied in chemical instruments and methods, catalysts, carbon compound catalysts, etc., to achieve the effect of great application value and high luminous quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] This embodiment provides a triplet annihilation agent (compound 1), and its structure and preparation flow diagram are as follows:
[0069]
[0070] The synthesis method is:
[0071] Weigh 300mg of 4,12-dibromo[2.2]paracyclic aromatic hydrocarbon, 47mg of tetrakis(triphenylphosphine)palladium, and 783mg of 4,4,5,5-tetramethyl-2-(10-tert-butylperylene-3 -base)-1,3,2-dioxaborolane was added to a 50mL reaction tube, 12mL of toluene was added, 3mL of ultrapure water dissolved with 87mg of sodium carbonate was added, and heated to reflux under nitrogen protection to react 24 After 1 hour, the raw material of 4,12-dibromo[2.2]paracyclic aromatic hydrocarbon was detected by TLC to disappear, that is, the reaction ended. The temperature was lowered to 25°C, the reaction liquid was poured into 50 mL of ethyl acetate for extraction, and the organic phase was dried with anhydrous sodium sulfate. The solvent was removed by rotary evaporation, and compound 1 was obtained by sil...
Embodiment 2
[0076] This embodiment provides a triplet annihilation agent (compound 1), and its structure and preparation flow diagram are as follows:
[0077]
[0078] The synthesis method is:
[0079] Weigh 300mg 4,12-dibromo[2.2]paracyclic aromatic hydrocarbon, 107mg triphenylphosphine, 13.56mg palladium acetate and 1070mg 4,4,5,5-tetramethyl-2-(10-tert-butylperylene- Add 3-yl)-1,3,2-dioxaborolane into a 50mL reaction tube, add 12mL of dioxane, add 3mL of ultrapure water dissolved with 130mg of sodium bicarbonate, under nitrogen protection, Heating to reflux, and reacting for 30 hours, the raw material of 4,12-dibromo[2.2]p-ring aromatic hydrocarbon disappeared by TLC, that is, the reaction ended. The temperature was lowered to 25°C, the solvent was removed by rotary evaporation, the mixture was dissolved in 50 mL of dichloromethane for extraction, and the organic phase was dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation, and compound 1 was obtain...
Embodiment 3
[0083] This embodiment provides a triplet annihilation agent (compound 2), and its structure and preparation flow diagram are as follows:
[0084]
[0085] The synthesis method is:
[0086]Weigh 300mg of 4,12-dibromo[2.2]paracyclic aromatic hydrocarbon, 69mg of tricyclohexylphosphine, 9mg of palladium chloride and 674mg of [1,1':4',1"-terphenyl]-4-ylboronic acid, Add to a 50mL reaction tube, add 12mL o-xylene, add 3mL ultrapure water dissolved with 316mg sodium bicarbonate, under nitrogen protection, heat to reflux, react for 18 hours, detect 4,12-dibromo[2.2] by TLC The p-ring aromatic hydrocarbon raw material disappears, that is, the reaction is over. Lowered to 25 ° C, the mixture is dissolved in 50 mL of ethyl acetate for extraction, and the organic phase is dried with anhydrous sodium sulfate. The solvent is removed by rotary evaporation, and compound 2 is obtained by silica gel column chromatography. The rate is 91%.
[0087] The NMR data are: 1 H NMR (500MHz, Chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com