Bifunctional antigen as well as preparation method and application thereof
A dual-function, antigenic technology, applied in the field of biomedicine, can solve the problem of no new coronavirus and pneumococcal vaccine antigen and its form, and achieve the effect of preventing and/or pneumococcus, low cost, and improving the level of neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
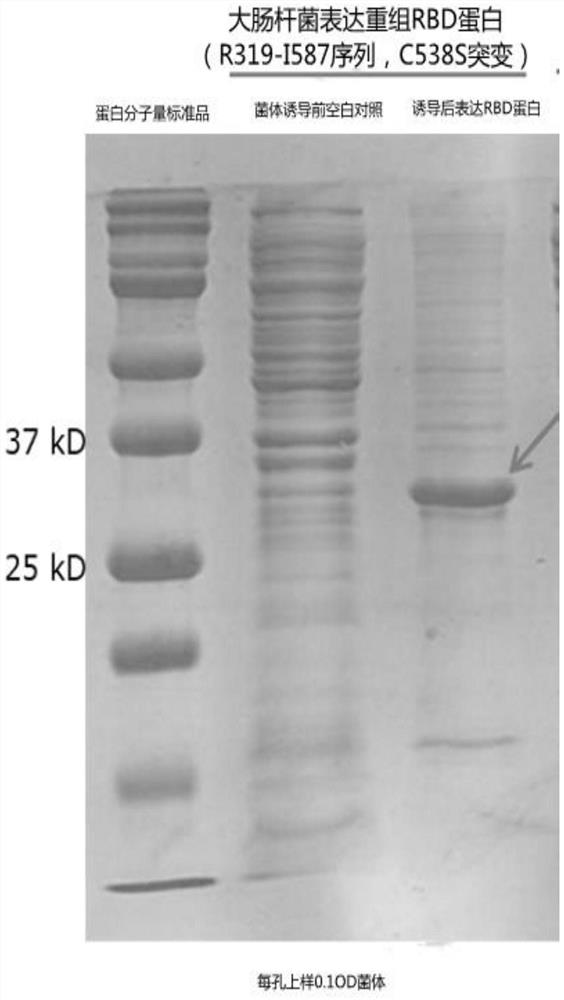
[0063] The preparation of the Escherichia coli engineering bacteria expressing SARS-CoV-2 recombinant RBD protein of embodiment 1
[0064] Entrusted Suzhou Jinweizhi to synthesize pET-28a plasmids containing SEQ ID NO:5 and SEQ ID NO:6 respectively, and the sequencing verification was correct.
[0065] The correct plasmid containing SEQ ID NO:5 was transformed into BL21(DE3) competent, and the single clone was inoculated into 3mL liquid LB medium containing 100ng / mL kanamycin, 220rpm, 30°C shaking overnight culture, as seeds.
[0066] Inoculate the seeds into TB medium containing 100ng / mL kanamycin at a ratio of 1:500, culture at 220rpm, shake at 37°C until OD 600 =2, add final concentration of 0.5mM IPTG, continue culturing for 4h, and collect the cells by centrifugation for purification.
[0067] Take 10OD cells and resuspend them with 1mL lysate (10mM Tris-HCl, pH 8.0), sonicate the bacteria, and conduct SDS-PAGE electrophoresis analysis of the total protein, supernatant a...
Embodiment 2
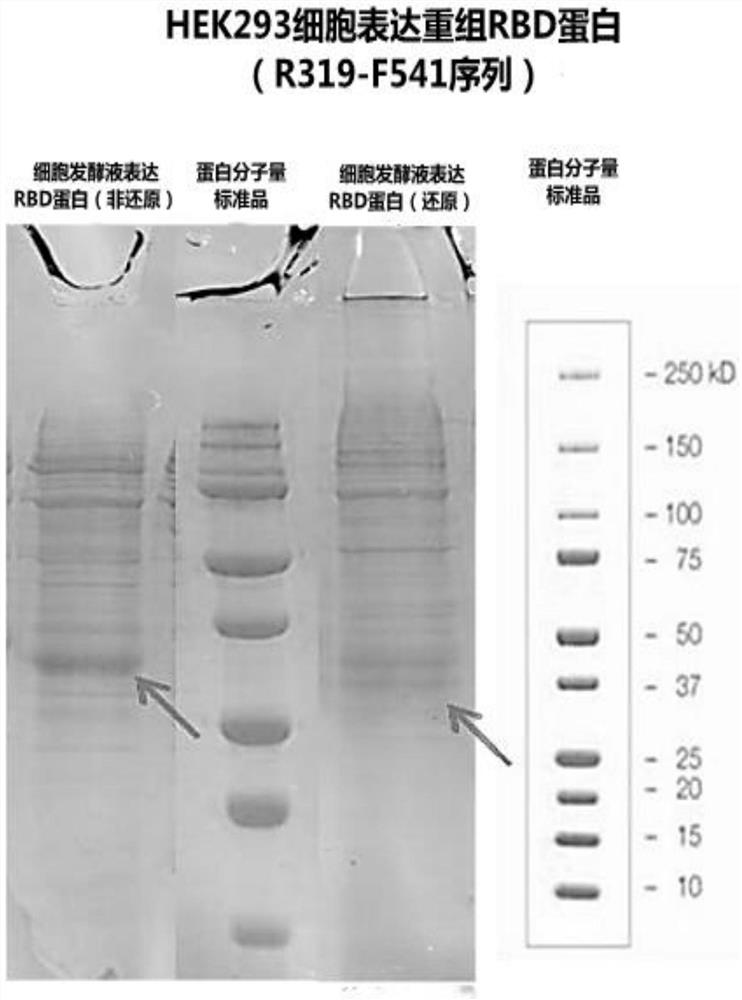
[0068] Example 2 Preparation of Expi293 recombinant cells expressing SARS-CoV-2 recombinant RBD protein (sequence is SEQ ID NO: 3), using QIAGEN plasmid extraction kit to extract the plasmid of the correct target gene for sequencing
[0069] According to the volume required for transfection, prepare a freshly subcultured Expi293 cell suspension (purchased from ATCC CRL-1573) to 2.0-2.5×10E6 cells / mL.
[0070] Prepare the transfection reagent-plasmid complex, namely liquid A (plasmid containing SEQ ID NO:6 synthesized in Example 1 / Opti-MEM=1 μg / 50 μl) and liquid B (PEI / Opti-MEM=5 μl / 50 μl) , Solution A and solution B were placed independently at room temperature for 10 minutes.
[0071] Add solution B to solution A dropwise, incubate at room temperature for 10 minutes, add cell suspension dropwise, and culture with shaking (115rpm, 36.8°C, 5% CO 2 ), 0.4mL KT Feed (Zhuhai Kairui) can be added after 24 hours, and the shaking culture is continued for 5 days to 7 days under the s...
Embodiment 3
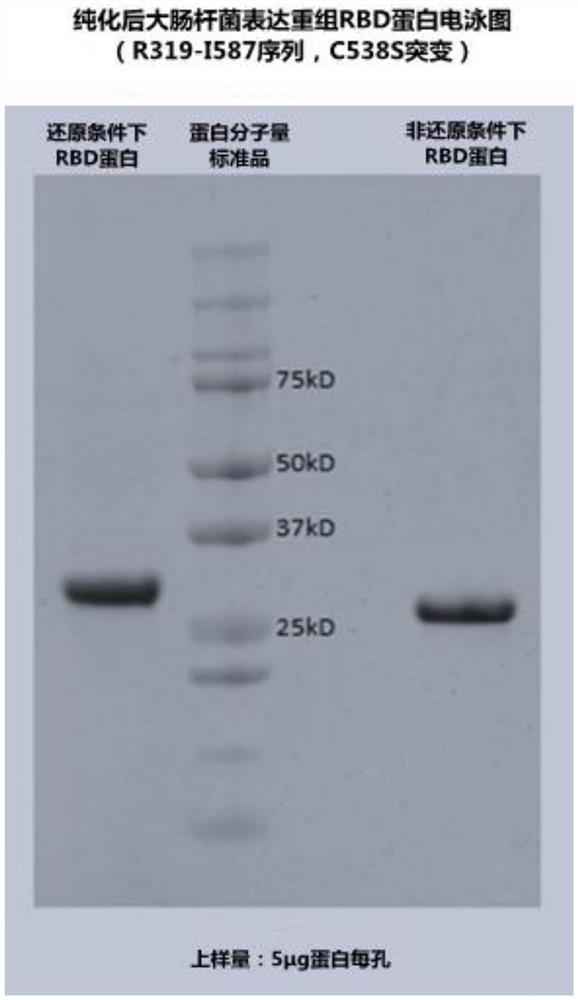
[0073] Example 3 Purification and identification of SARS-CoV-2 recombinant RBD protein (sequence is SEQ ID NO: 2) Escherichia coli or cell expression
[0074] The Escherichia coli expression bacterium containing SEQ ID NO: 5 in Example 1 was resuspended in the Tris buffer solution of pH 8.0 with 1:20 (W / V), and was broken for 3 times with a high-pressure homogenizer 900bar* Inclusion bodies were obtained by centrifugation. Dissolve and denature the inclusion bodies with a solution (20mM ethylene glycol, 8M Urea, 50mM DTT pH8.0) and add refolding solution (20mM Tris, pH8.0, 3M urea, 0.05mM cystamine, 1mM cysteamine) for refolding 20 hours. Adjust the pH value of the renaturation supernatant to 7, centrifuge at 10000×g for 1 hour, take the supernatant and purify it through Cytiva SP Sepharose Fast Flow at a flow rate of 1-100cm / h to remove the adsorbed HCP (host cell residual protein, Host cell protein) Purify the target protein with polymers; collect the peak of the target RB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com