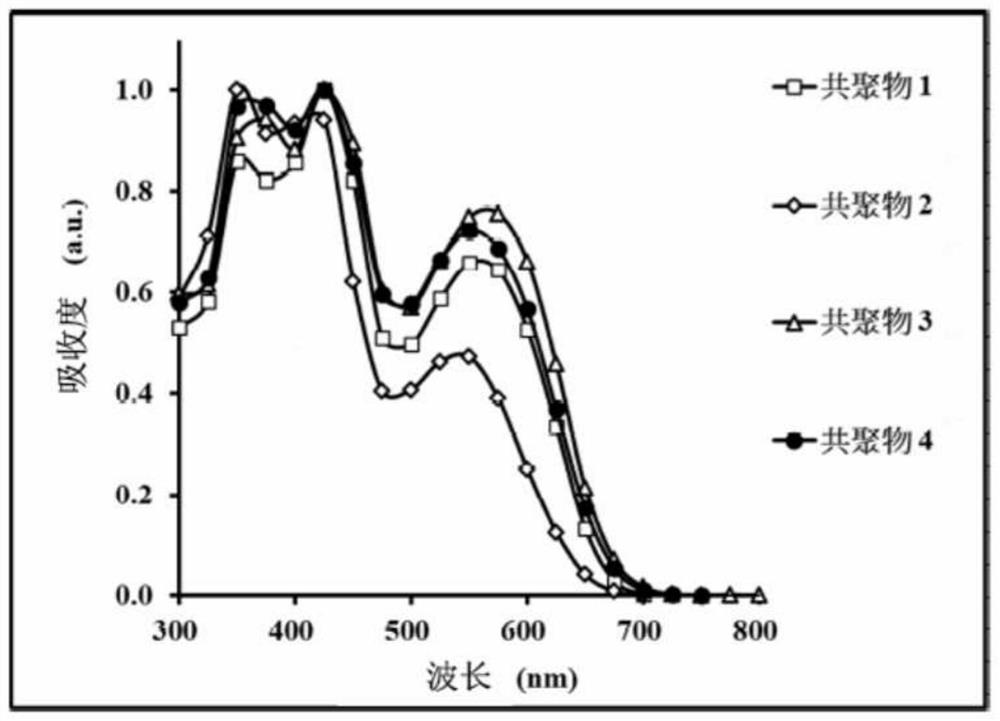
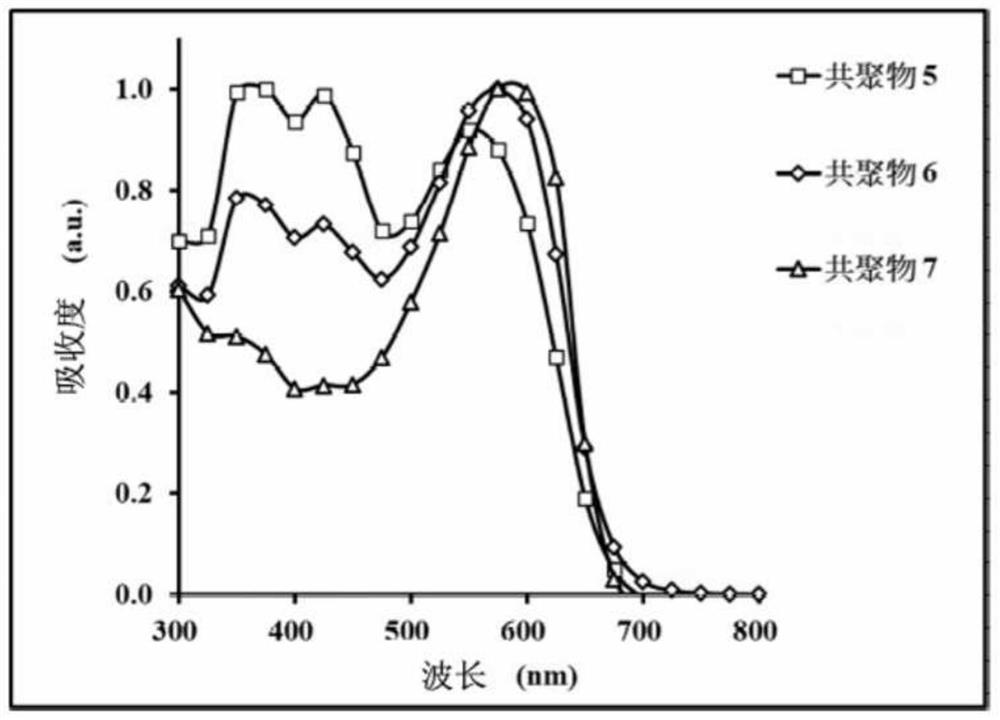
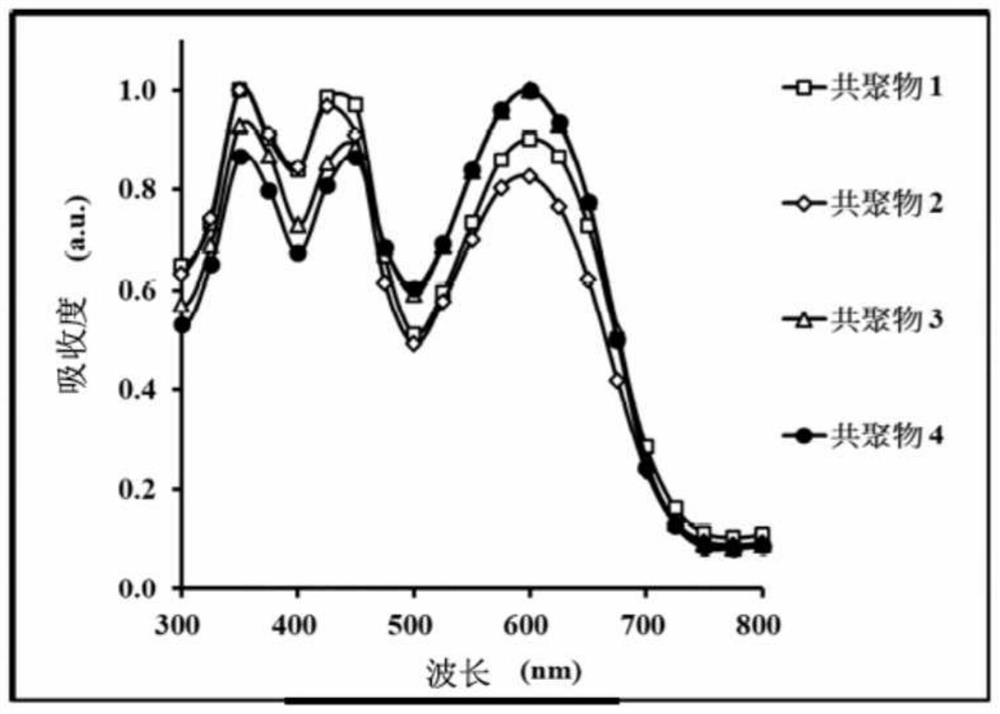
Copolymer and organic photovoltaic element
A technology of copolymer and chemical formula, applied in the field of organic photovoltaic elements, can solve the problems of weak absorption in the visible light region, high price, difficult structure modification and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Preparation of copolymers
[0137] The copolymer of Example 1 comprises the structure shown below, wherein X is F or Cl.
[0138]
[0139] The copolymer of Example 1 was prepared according to the following steps 1 to 7. It should be noted that X in all the chemical formulas in steps 1 to 7 is F or Cl.
[0140] Step 1 (preparation of compound 2)
[0141]
[0142] Compound 1 (10 mmol) and sodium borohydride (NaBH 4 ) (25mmol) into a 250mL reaction flask, add 100mL of absolute ethanol (EtOH), heat to 78°C and stir for 1 hour. Next, after adding water and extracting with dichloromethane and drying over anhydrous magnesium sulfate, the solid was removed by filtration. The filtrate was concentrated to remove the solvent to obtain compound 2 as a dark brown solid.
[0143] Step 2 (preparation of compound 4)
[0144]
[0145] Compound 3 (12 mmol) was fed into a 250 mL reaction flask under nitrogen, and 150 mL of anhydrous tetrahydrofuran (THF) was added, and the ...
preparation Embodiment 1
[0160]
[0161] Compound 8 (1mmol), compound 9 (1mmol), tris(2-furyl)phosphine[(o-toly) 3 P] (0.12mol), three (dibenzylideneacetone) dipalladium [Pd 2 (dba) 3 ] (0.03mol) was charged in a 100mL reaction flask. Next, 40 mL of anhydrous chlorobenzene (PhCl) was added, stirred at 130° C. for 1 hour, the reaction was cooled to room temperature, and the contents of the reaction bottle were poured into methanol to precipitate a solid. The precipitate was collected by filtration, and the solid was subjected to Soxhlet extraction with methanol, acetone and chloroform sequentially. Finally, the chloroform residue was poured into methanol for reprecipitation, and the precipitate was collected by filtration and vacuum-dried to obtain a red-black copolymer (Example 1).
Embodiment 2
[0163] Preparation of copolymers
[0164] The copolymer of Example 2 comprises the structure shown below, wherein X is F or Cl.
[0165]
[0166] The steps of embodiment 2 are similar to those of embodiment 1, the difference being that step 7 of embodiment 1 is replaced by the following step 7' in embodiment 2. It should be noted that X in all the chemical formulas in step 7' is F or Cl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com