A kind of detection method of degradation impurity aldol dimer content in oxycodone liquid preparation
A technology of aldol dimer and liquid preparations, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of affecting the sensitivity of detection samples, not easy to separate the main component oxycodone, peak tailing, etc., and achieve the elimination of impurities Accurate and reliable quantification, improved accuracy, and improved peak parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
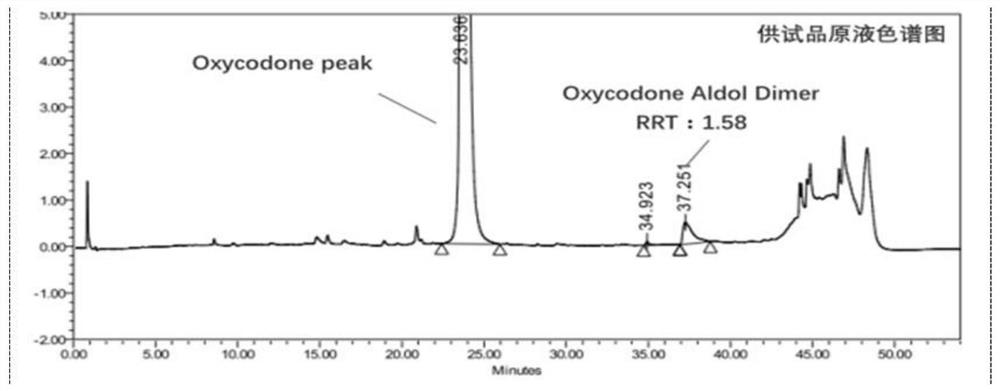
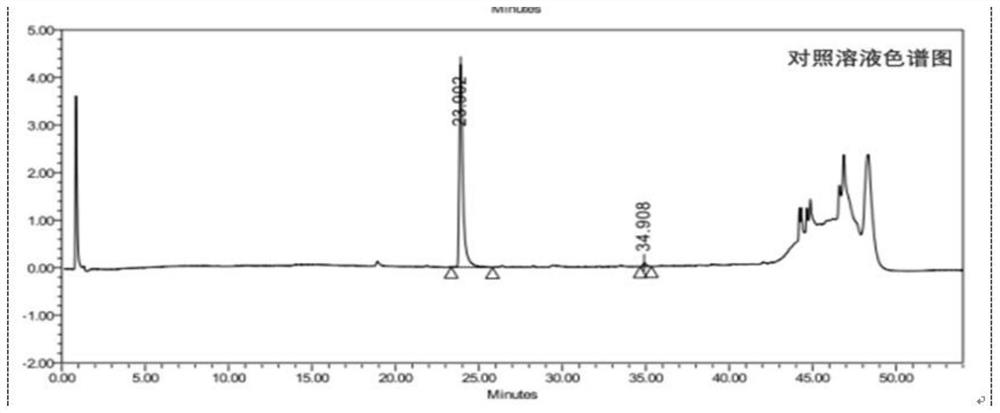
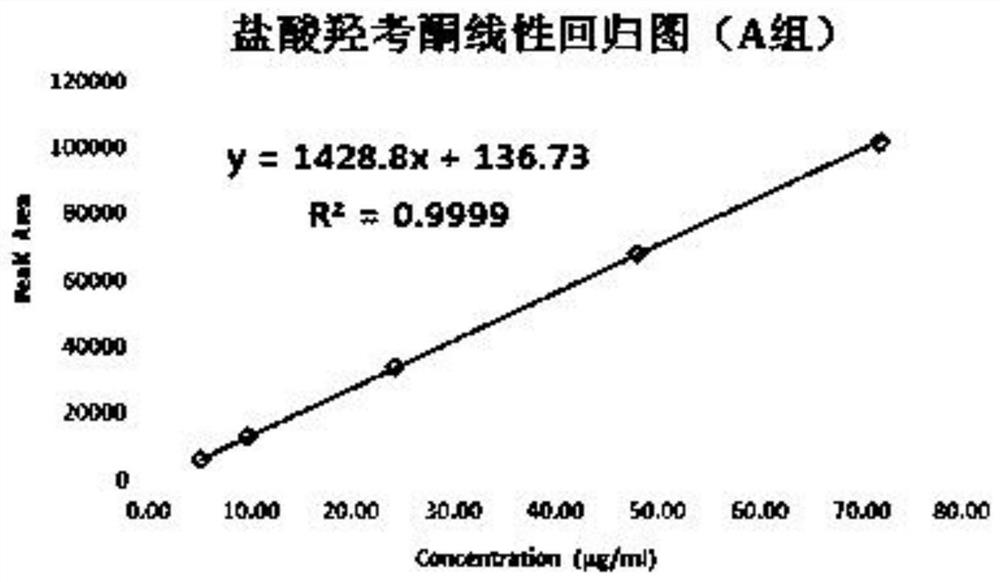
[0114] Test solution: commercially available oxycodone hydrochloride injection (marked amount: 100.5%, specification: 10mg: 1mL);
[0115] Reference substance solution: the test solution is diluted with 0.85% phosphoric acid aqueous solution to a solution containing 50 μg of oxycodone hydrochloride per 1 mL, as the reference substance solution;
[0116] Sensitivity solution: dilute the test solution with 0.85% phosphoric acid aqueous solution to a solution containing 1.5 μg of oxycodone hydrochloride per 1 mL, as a sensitivity solution;
[0117] High performance liquid chromatography: Agilent 1290 chromatography;
[0118] Chromatographic column: high-performance liquid chromatography column with octylsilane bonded silica gel as filler, and the column specification is WatersC8 4μm, 3.9*150mm chromatographic column;
[0119] Mobile phase: sodium octane sulfonate buffer solution-acetonitrile (volume ratio 1100:150) as mobile phase A, acetonitrile-water (volume ratio 400:100) a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com