Method for recycling lithium from positive electrode material of waste lithium ion battery
A technology for lithium ion batteries and cathode materials, which is applied in the field of waste lithium ion battery recycling, can solve the problems of short comprehensive recycling process, difficult subsequent waste liquid treatment, and no closed-loop process, so as to improve recovery rate and purity, and be convenient and easy to operate. Achieving and extracting the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
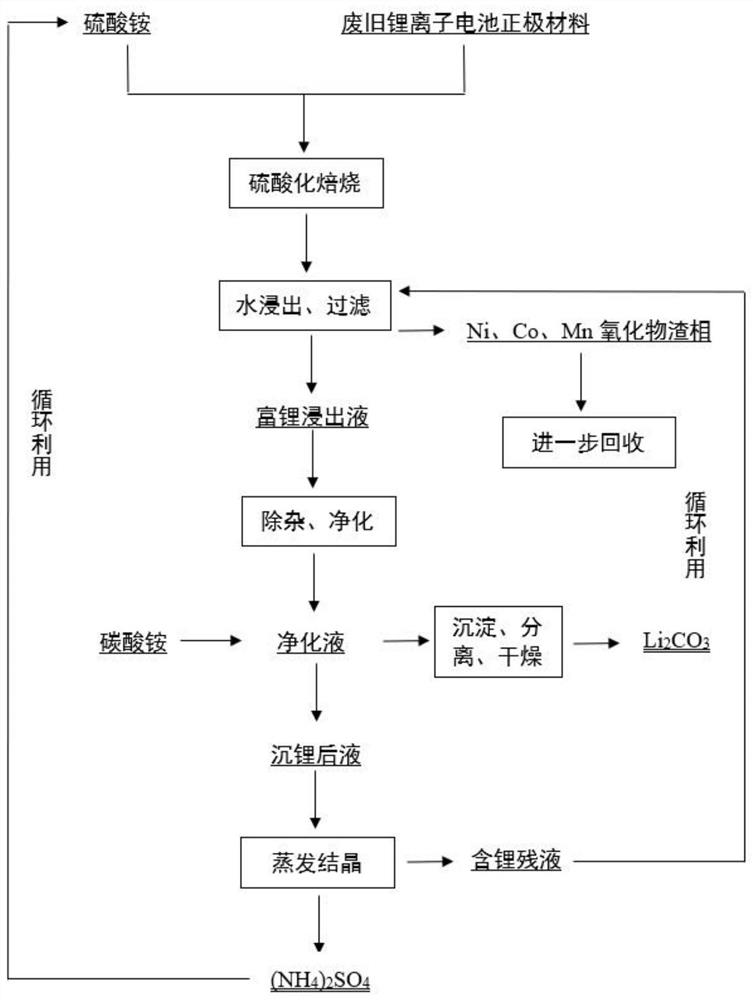
[0037] A method for reclaiming lithium from waste lithium-ion battery cathode materials, comprising the following steps:
[0038] (1) Combine waste nickel-cobalt lithium manganese oxide cathode material with (NH 4 ) 2 SO 4 press n (NH4)2SO4 :n Li = 1:1 After fully mixing, carry out sulfate roasting at 500°C for 60 minutes, the waste positive electrode material is decomposed, and lithium ions are extracted from the crystal structure.
[0039] (2) Disperse the calcined product in deionized water (solid-to-liquid ratio 3:1), water leaching temperature is 45°C, leaching time is 25min, after leaching is completed, filter and separate to obtain lithium-rich leachate and transition metal slag phase.
[0040] After sampling and diluting the lithium-rich leaching solution, an inductively coupled plasma emission spectrometer was used to analyze and measure the mass fraction of lithium in it to be 15g / L, and Al and Fe to be less than 0.6g / L. The leaching slag sample was taken, and t...
Embodiment 2
[0042] A method for reclaiming lithium from waste lithium-ion battery cathode materials, comprising the following steps:
[0043] (1) Combine waste nickel-cobalt lithium manganese oxide cathode material with (NH 4 ) 2 SO 4 press n (NH4)2SO4 :n Li = 2:1 After fully mixing, sulfuration roasting is carried out at 600°C for 80 minutes, the waste positive electrode material is decomposed, and lithium ions are extracted from the crystal structure.
[0044] (2) The roasted product was dissolved in deionized water (solid-to-liquid ratio 3:1), the water leaching temperature was 45°C, and the leaching time was 25 minutes. After filtration and separation, the lithium-rich leaching solution and transition metal slag phase were obtained.
[0045] After sampling and diluting the lithium-rich leaching solution, an inductively coupled plasma emission spectrometer was used to analyze and measure the mass fraction of lithium in it to be 14.5g / L, and Al and Fe to be less than 0.5g / L. The le...
Embodiment 3
[0047] A method for reclaiming lithium from waste lithium-ion battery cathode materials, comprising the following steps:
[0048] (1) Combine waste lithium cobaltate cathode material with (NH 4 ) 2 SO 4 press n (NH4)2SO4 :n Li = 2:1 After fully mixing, sulfuration roasting is carried out at 600°C for 80 minutes, the waste positive electrode material is decomposed, and lithium ions are extracted from the crystal structure.
[0049] (2) The roasted product was dissolved in deionized water (solid-to-liquid ratio 3:1), the water leaching temperature was 40°C, and the leaching time was 30 minutes. After filtration and separation, the lithium-rich leaching solution and transition metal slag phase were obtained.
[0050] After sampling and diluting the lithium-rich leaching solution, an inductively coupled plasma emission spectrometer was used to analyze and determine that the mass fraction of lithium was 15.8 g / L, and Al and Fe were less than 0.5 g / L. The leaching slag sample w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com