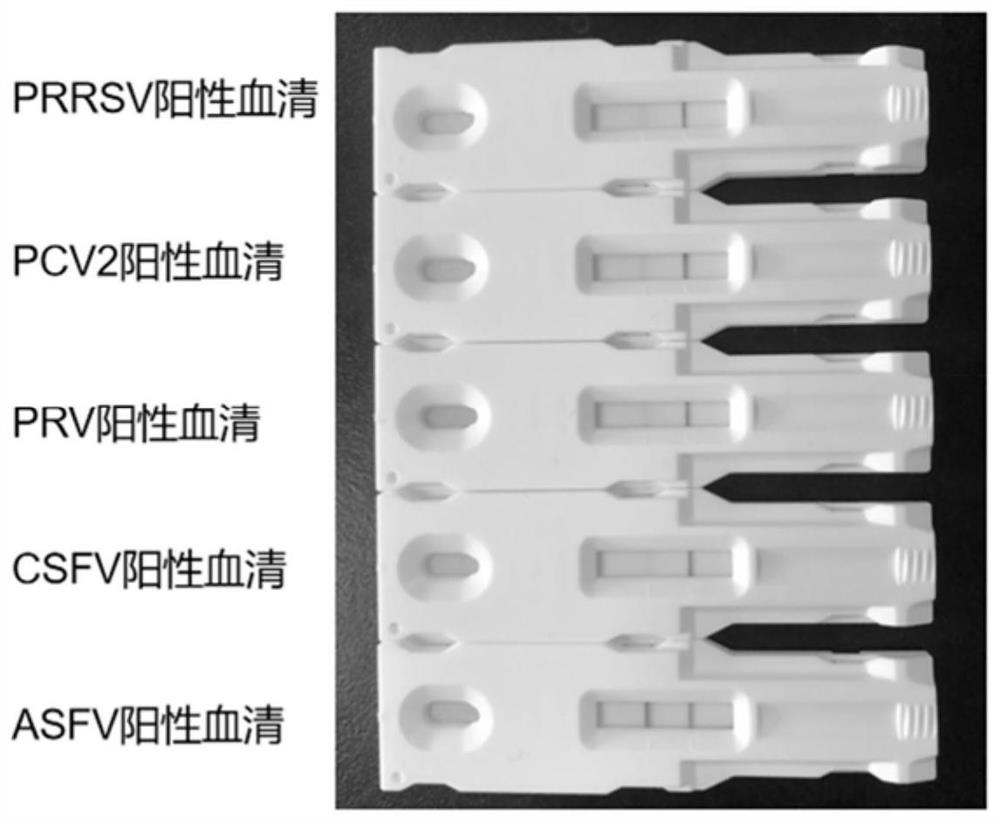
African swine fever virus p72 recombinant protein and colloidal gold immunochromatography test paper constructed by same
A technology of African swine fever virus and immunochromatographic test paper, which is applied in the field of virus epidemic diagnosis and animal quarantine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1p72
[0050] Expression of embodiment 1p72 recombinant protein
[0051] Using the African swine fever virus ASFV-SY18 sequence published in GenBank (GenBank accession number: MH766894) as a template, the p72 gene fragment was synthesized by Beijing Qingke Biotechnology Co., Ltd.
[0052] The following expression primers were designed to amplify the p72 gene:
[0053] Upstream primer: 5'-GAGCTCGGTACCCTCGAGTTAGGTACTGTAACGCAG-3';
[0054] Downstream primer: 5'-CTGCAGGTCGACAAGCTTTCAATGGCATCAGGAGGAGCT-3'.
[0055] PCR amplification system (total system 25 μL) includes:
[0056]
[0057] The specific PCR amplification program includes: 98°C for 5min; 98°C for 40s, 55°C for 40s, 68°C for 3min, 35 cycles; 68°C for 10min.
[0058] After the amplified product was subjected to 1% agarose gel electrophoresis, the Omega gel recovery kit was used for gel recovery, and the gel recovery product was used directly or stored at -20°C for future use.
Embodiment 2
[0067] Embodiment 2 recombinant protein purification
[0068] The p72 recombinant protein expressed in the supernatant in Example 1 was affinity-purified on a nickel column prepacked column and then further purified by molecular sieves. The main operation steps included: taking 10 mL of the protein supernatant and adding it to 800 μL of nickel column, mixing it thoroughly, and placing it at 4°C After incubation for 12 h, centrifuge at 3000 rpm and 4°C for 10 min, discard the supernatant, and wash with 10 mM, 20 mM, 50 mM, and 100 mM imidazole solutions at pH = 7.4, respectively. The amount of each gradient washing solution is 30 mL. After washing, 5 mL of 250 mM imidazole solution with pH=7.4 was used for elution, and the collected eluate was passed through a molecular sieve of a protein purifier for further protein purification.
Embodiment 3
[0069] Example 3 colloidal gold particle labeling purified p72 recombinant protein
[0070] Take 0.01% HAuCl 4 Put 100mL of aqueous solution in a beaker, heat to boil fully, add 1mL of 1% trisodium citrate aqueous solution, continue to heat and boil for 5min, until the solution is orange-red, and the prepared colloidal gold particles are about 30nm, and stored at 4°C.
[0071] Take 500 μL of the above colloidal gold solution, add 1 μL, 2 μL, 4 μL, 8 μL and 16 μL of K at a concentration of 0.2 mol / L, respectively. 2 CO 3 The solution was mixed well, and then 0.4 μg, 0.8 μg, 1.6 μg, 3.2 μg and 6.4 μg of the above-mentioned purified p72 recombinant protein were added respectively, mixed well and then allowed to stand at room temperature for 20 minutes. Continue to add 50 μL of BSA with a concentration of 10%, mix well, block at room temperature for 10 minutes, centrifuge at 4°C, 1000rpm for 10min to remove excess colloidal gold, absorb the supernatant, and centrifuge at 4°C, 75...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com