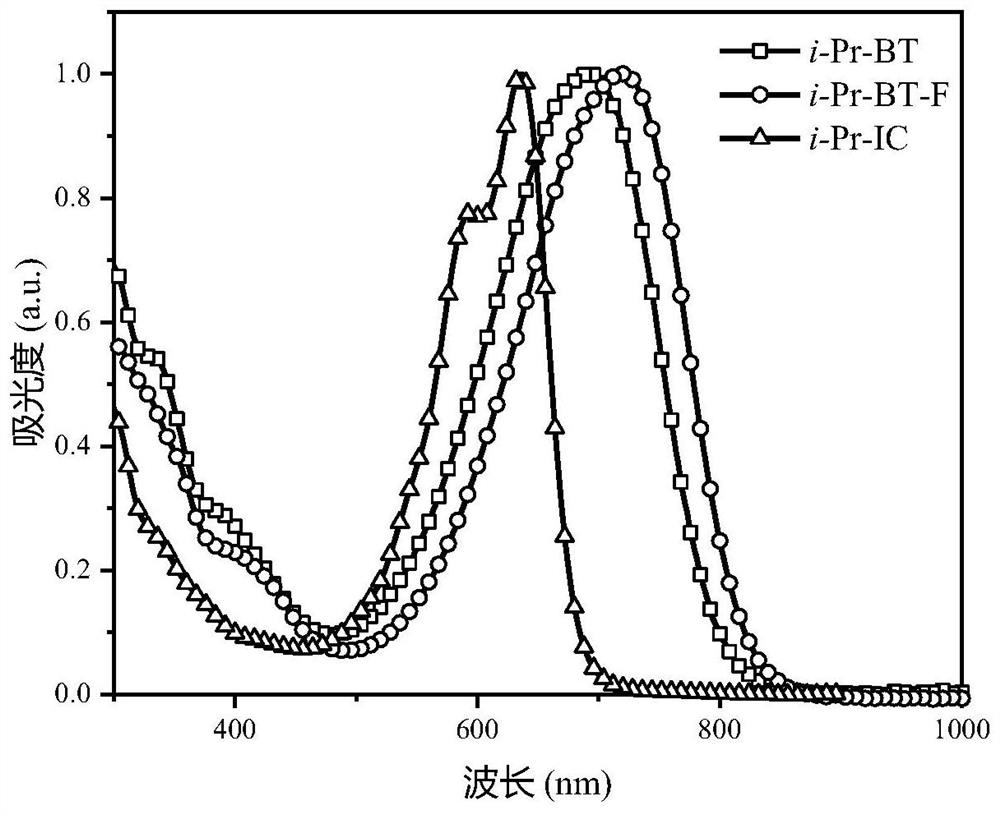
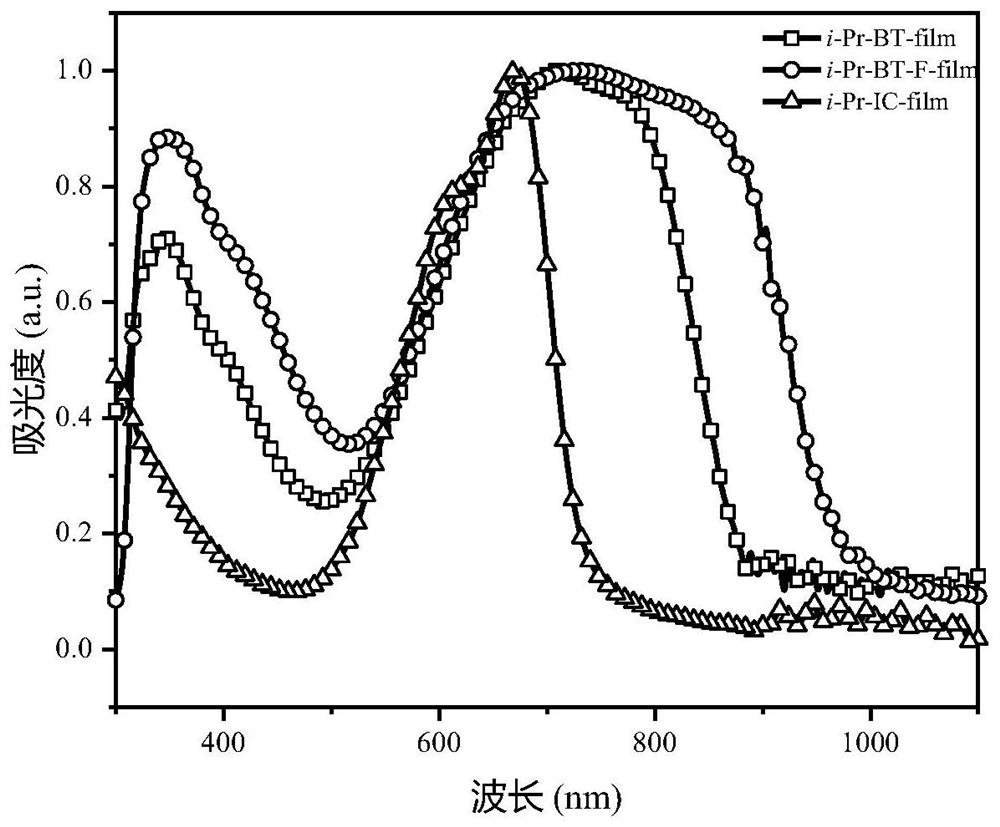
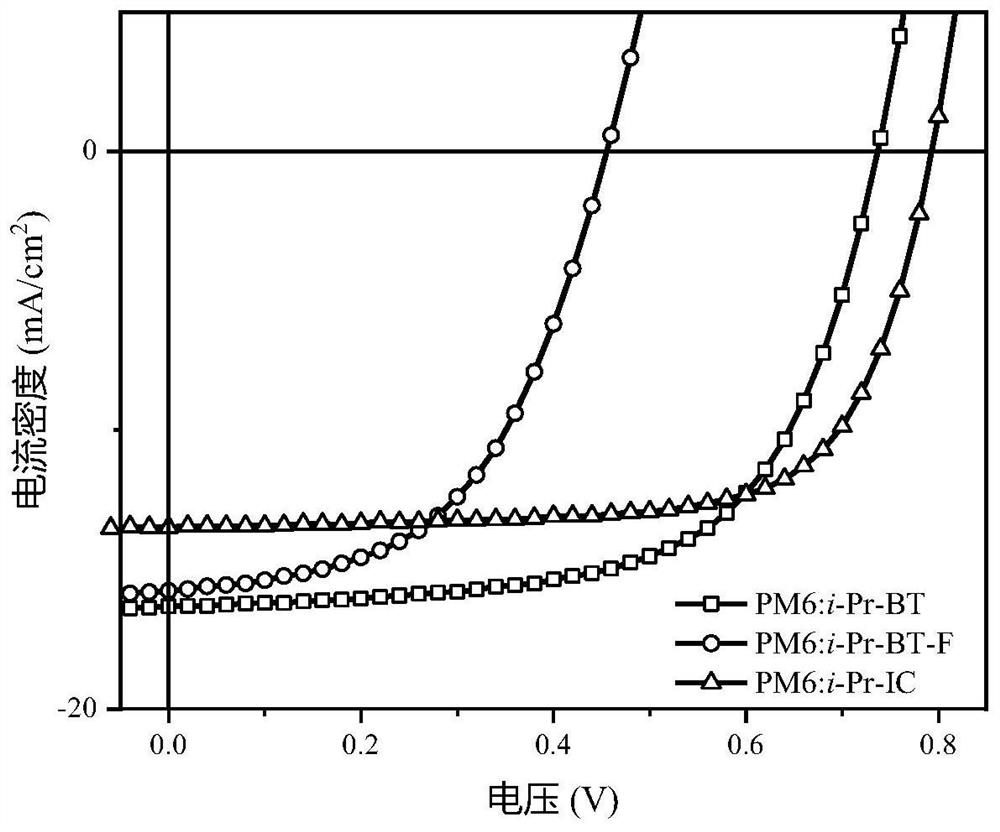
Non-fullerene acceptor material as well as preparation method and application thereof
A technology of non-fullerene acceptors and acceptor materials, applied in the field of non-fullerene acceptor materials, can solve the problems of complex synthesis and difficult structure adjustment of non-fullerene acceptor materials, and achieve good solubility, electronic The effect of high mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the synthesis of compound Me-BT
[0094]
[0095] Step 1: Synthesis of Compound 2-1
[0096] Magnesium chips (2.92g, 120mmol) were added to a 250mL three-necked bottle, the argon gas was replaced three times, a grain of iodine was added to the bottle under argon flow, and then 30mL of anhydrous tetrahydrofuran was added. Mesitylbromobenzene (23.89g, 120mmol) was dissolved in 60mL of anhydrous tetrahydrofuran, and 20mL of the solution was first added to the reaction system, and the reaction was initiated by heating until the reaction liquid became colorless, and the reaction initiation was completed, and continued in a slightly boiling state. Add the remaining solution dropwise, heat to reflux after the dropwise addition to complete the reaction of magnesium, and cool down for later use. Take another 250mL three-necked bottle, add palladium acetate (269.4mg, 1.2mmol) and 2-dicyclohexylphosphino-2'-(N,N-dimethylamine)-biphenyl (2.29g, 4.8mmol) to it and ...
Embodiment 2
[0113] Embodiment 2: the synthesis of compound i-Pr-BT
[0114]
[0115] Step 1: Synthesis of compounds 2-6
[0116]In the 500mL three-necked flask, add triisopropylbromobenzene (14.16g, 50mmol), 3-thiophene boronic acid (12.80g, 100mmol), potassium phosphate (21.23g, 100mmol), tridibenzylideneacetone dipalladium (458mg, 0.5mmol) and 2-bicyclohexylphosphine-2',6'-dimethoxybiphenyl (821mg, 2mmol), the argon gas was replaced three times, and 100mL anhydrous toluene and 100mL anhydrous tetrahydrofuran were added as solvents, and three liquid Nitrogen freezing-pumping-thawing cycle removes dissolved oxygen in the solvent and heats to reflux for reaction. The reaction was monitored by TLC, and the developing solvent was petroleum ether. Extracted with dichloromethane, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography with petroleum ether as the eluent to obtain 14.10 g of compound 2-6 as a ...
Embodiment 3
[0133] Embodiment 3: the synthesis of compound i-Pr-IC and i-Pr-IC-F
[0134]
[0135] Step 1: Synthesis of Compound 2-11
[0136] Add compound 2-8 (1.14g, 2mmol), 30mL 1,2-dichloroethane and 1.9mL phosphorus oxychloride to a 100mL three-necked flask, add 1.6mL N,N-dimethylformamide dropwise, and heat Reflux reaction for 24h. After the reaction was complete, it was extracted with dichloromethane, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography. The eluent used was petroleum ether:dichloromethane=1:1, and compound 2- 11 Yellow solid 970.0 mg, yield 77.4%.
[0137] 1 H NMR (400MHz, CDCl 3 )δ: 9.74(s,1H),7.39(s,1H),7.14(s,2H),3.00(p,J=6.9Hz,1H),2.48(p,J=6.9Hz,2H),1.35( d,J=6.9Hz,6H),1.05(t,J=6.8Hz,12H).MS(EI)m / z:627.3(M+H + ).
[0138] Step 2: Synthesis of Compounds i-Pr-IC and i-Pr-IC-F
[0139]Add compound 2-11 (200 mg, 0.32 mmol), 30 mL chloroform, and 1.9 mL pyridine into a 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com