Thiadiazole amide compound and application thereof
A technology of thiadiazole amide and compound is applied in the application field of preparing drugs for the treatment of prostate cancer, which can solve the problems of multidrug resistance, reduce drug efficacy, limit clinical treatment options and benefits, etc., and achieve high antagonistic androgen receptors. The effect of in vivo transcriptional activity, good biological activity, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
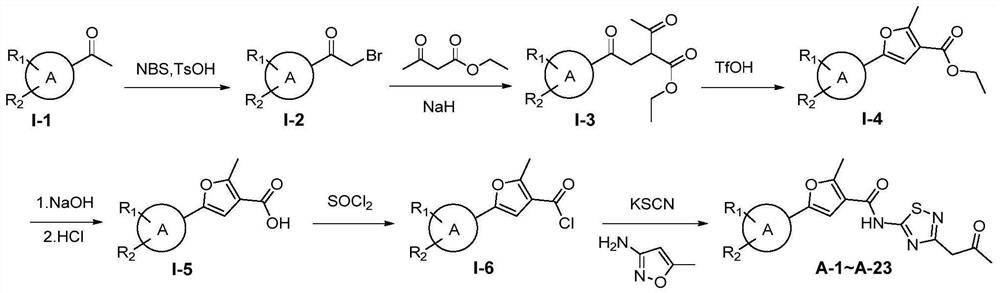
[0121] Embodiment 1: Synthesis of Target Molecules A-1~A-23
[0122] The synthetic routes of compounds A-1~A-23 are as follows: figure 1 shown.
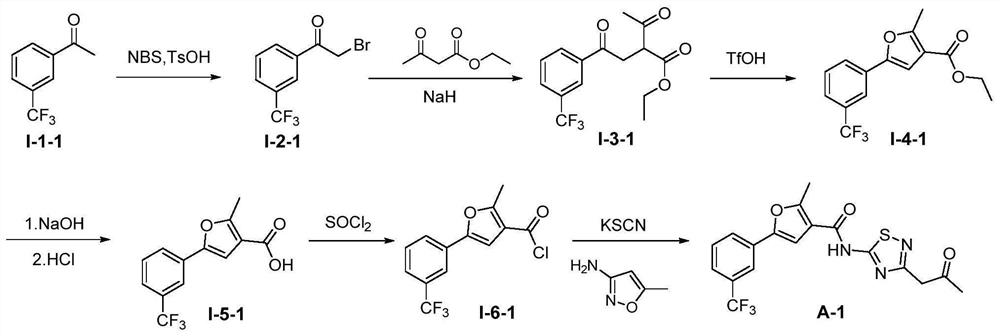
[0123] 2-Methyl-5-(3-(trifluoromethyl)phenyl)-N-(3-(2-oxopropyl)-1,2,4-thiadiazol-5-yl)furan- 3-Formamide (Compound A-1)
[0124] The specific synthetic route is as figure 2 shown.
[0125] (a) Synthesis of 2-bromo-1-(3-trifluoromethylphenyl)ethan-1-one (compound I-2-1)
[0126] Dissolve NBS (1.04g, 5.9mmol) and p-toluenesulfonic acid (458mg, 2.7mmol) in acetonitrile (20mL), add m-trifluoromethyl acetophenone (I-1-1, 1.00g, 5.3mmol) , Stir the reaction at 55°C for 1h. Add an appropriate amount of ethyl acetate to the system, wash with water and saturated brine successively, and wash the organic layer with anhydrous Na 2 SO 4 Dried and concentrated. The residue was subjected to column chromatography to obtain a light yellow oily liquid with a yield of 88%; ESA-MS: m / z=267.0[M+H] + .
[0127] (b) Synthesis of ethyl 2-acetyl...
Embodiment 2
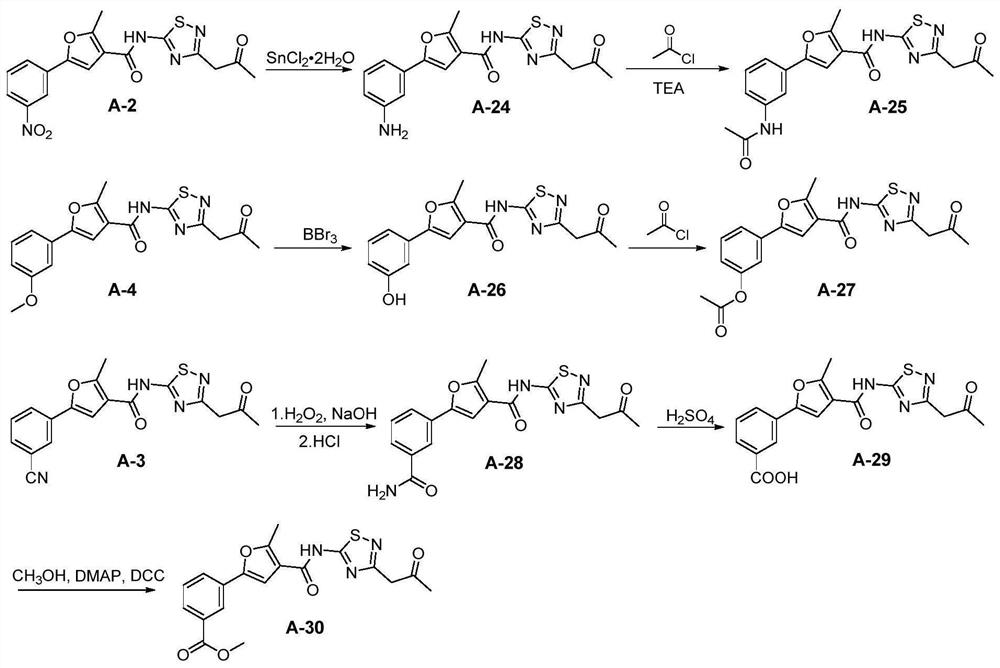
[0181] Embodiment 2: the synthesis of compound A-24~A-30
[0182] Compounds A-24~A-30 synthetic routes are as follows: image 3 shown.
[0183] 2-methyl-5-(3-aminophenyl)-N-(3-(2-oxopropyl)-1,2,4-thiadiazol-5-yl)furan-3-carboxamide ( Compound A-24)
[0184] Compound A-2 (215mg, 0.56mmol) was dissolved in ethanol and water (3:1, 12mL), iron powder (225mg, 4.0mmol) and ammonium chloride (89mg, 1.7mmol) were added, and reacted at 75°C for 6h. Add saturated sodium bicarbonate solution to the system to adjust the pH to weakly alkaline, then add an appropriate amount of ethyl acetate, wash with water and saturated brine in sequence, and wash the organic layer with anhydrous Na 2 SO 4 After drying and concentration, the residue was subjected to column chromatography to obtain a light yellow solid with a yield of 72%; 1 H NMR (500MHz, DMSO-d 6 ): δ13.12(s,1H),7.54(s,1H),7.10(t,J=8.0Hz,1H),6.87(t,J=2.0Hz,1H),6.80-6.76(m,1H) ,6.57-6.52(m,1H),5.30(s,2H),4.04(s,2H),2.68(s,3H),2.21(...
Embodiment 3
[0197] Embodiment 3: the synthesis of compound A-31~A-33
[0198] 2-Methyl-5-(pyridin-3-yl)-N-(3-(2-oxopropyl)-1,2,4-thiadiazol-5-yl)furan-3-carboxamide ( Compound A-31)
[0199] Synthetic route such as Figure 4 shown.
[0200] (a) Synthesis of methyl 2-methyl-5-(pyridin-3-yl)furan-3-carboxylate (compound II-2-1)
[0201] 3-bromopyridine (II-1-1, 122μL, 1.27mmol), 2-methylfuran-3-carboxylate methyl ester (317μL, 2.53mmol), potassium acetate (248mg, 2.53mmol) and palladium acetate (0.1% equivalent) was added to DMA (5 mL), and stirred overnight at 150°C. Add an appropriate amount of ethyl acetate to the system, wash with water and saturated brine successively, and wash the organic layer with anhydrous Na 2 SO 4 Drying and concentration, the residue was subjected to column chromatography to obtain a yellow solid with a yield of 88%; ESA-MS: m / z=218.1[M+H] + .
[0202] (b) Synthesis of 2-methyl-5-(pyridin-3-yl)furan-3-carboxylic acid (compound II-3-1)
[0203] A solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com