Preparation method of self-supporting coralline-like array structure electrode
An array structure, coral-like technology, applied in the field of catalyst preparation, can solve problems such as pollution, urea abuse, energy shortage, etc., achieve broad application prospects, stable product quality, and improve the effect of urea pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
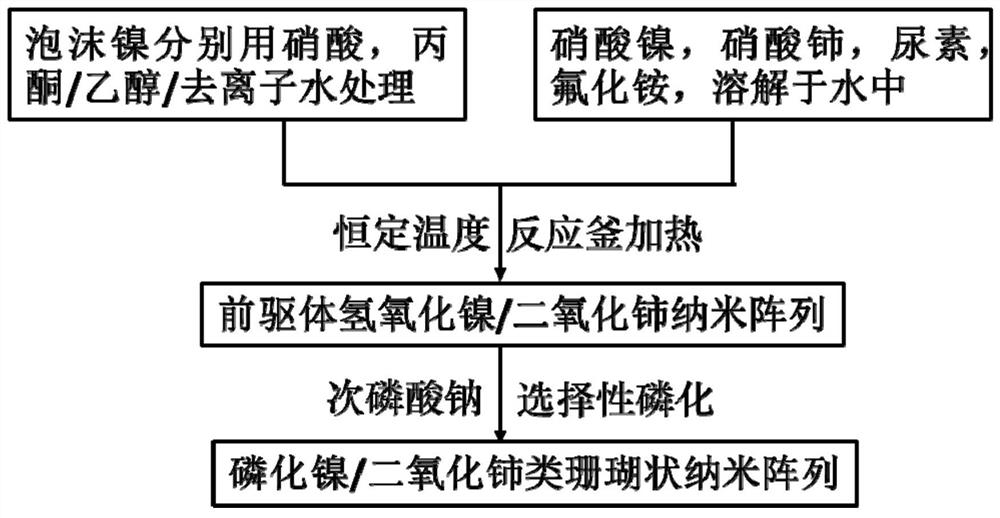
[0025] (1) Preparation of the precursor array structure: use nickel foam as the substrate, rinse with 5% dilute nitric acid, ethanol and deionized water three times, remove the surface organic impurities and oxide layer, dry naturally, weigh 0.9g of nickel nitrate hexahydrate , 0.4g of cerium nitrate hexahydrate, 1.2g of urea, and 0.4g of ammonium fluoride were dispersed in 30mL of deionized water, transferred to a 50mL reaction kettle, stirred evenly and dissolved, and the nickel foam was transferred to it and packaged, transferred to an oven, and kept at a constant temperature Keep at 100°C for 8 hours, then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide / ceria nanosheet array.
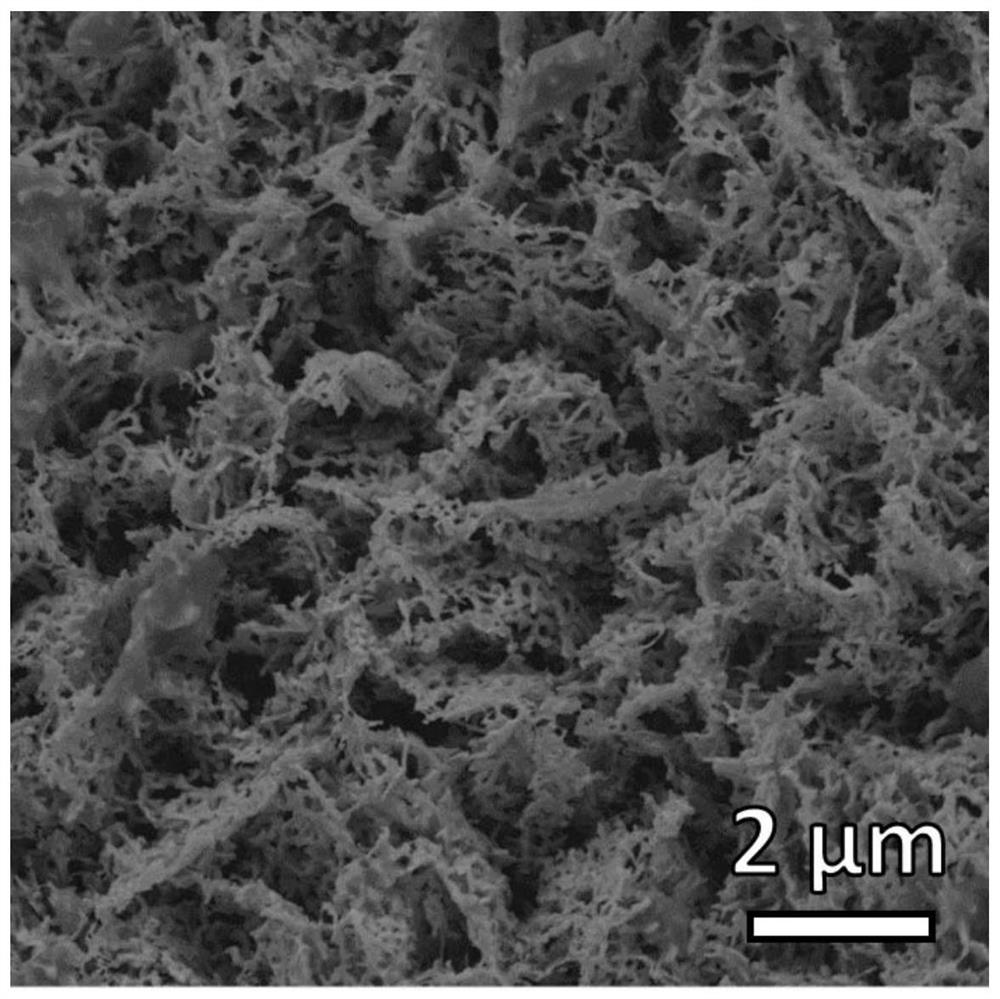
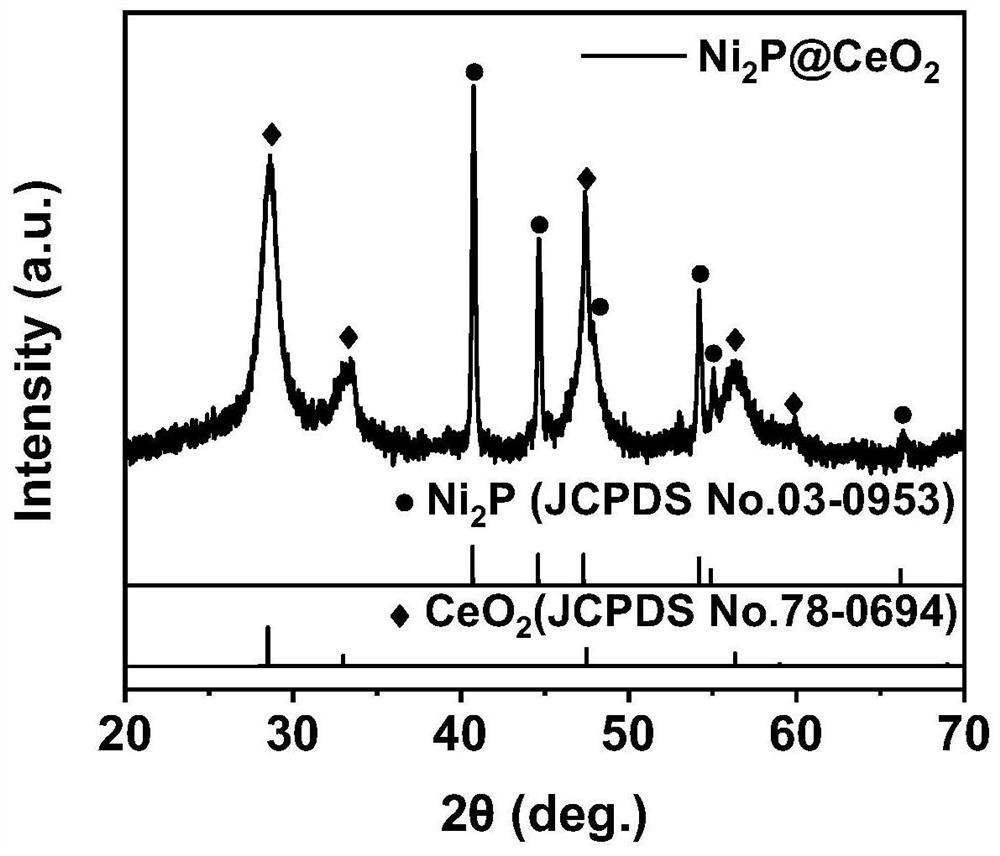
[0026] (2) Preparation of nickel phosphide / ceria-like coral-like nano-arrays: put the nickel hydroxide / ceria nanosheet arrays obtained in step (1) into a porcelain bo...
Embodiment 2
[0028] (1) Preparation of the precursor array structure: use nickel foam as the substrate, rinse with 15% dilute nitric acid, ethanol and deionized water five times, remove the surface organic impurities and oxide layer, dry naturally, weigh nickel nitrate hexahydrate 1.4 g, 0.65g of cerium nitrate hexahydrate, 1.8g of urea, and 0.7g of ammonium fluoride were dispersed in 30mL of deionized water, transferred to a 50mL reaction kettle, stirred and dissolved evenly, and the nickel foam was transferred to it and packaged, transferred to an oven, Maintain a constant temperature of 120°C for 10 hours, then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide / ceria nanosheet array.
[0029] (2) Preparation of nickel phosphide / ceria-like coral-like nano-arrays: put the nickel hydroxide / ceria nanosheet arrays obtained in step (1) into a porcelain boat, put 8...
Embodiment 3
[0031] (1) Preparation of the precursor array structure: use nickel foam as the substrate, rinse with 30% dilute nitric acid, ethanol and deionized water five times, remove the surface organic impurities and oxide layer, dry naturally, weigh nickel nitrate hexahydrate 1.8 g, 0.9g of cerium nitrate hexahydrate, 2.4g of urea, and 0.9g of ammonium fluoride were dispersed in 80mL of deionized water, transferred to a 100mL reaction kettle, stirred and dissolved evenly, and the nickel foam was transferred to it and packaged, transferred to an oven, Keep the constant temperature at 100°C for 6 hours, then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide / ceria nanosheet array.
[0032] (2) Preparation of nickel phosphide / ceria-like coral-like nano-arrays: put the nickel hydroxide / ceria nanosheet arrays obtained in step (1) into a porcelain boat, put 100 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com