Fluorinated electroactive cross-linkable polymers comprising photoactive groups
A technology of fluorination and radicals, applied in the field of electroactive fluoropolymers, can solve problems such as deterioration of electroactive properties and complex preparation of polymer films, and achieve the effect of high saturation polarization and high dielectric constant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] In the first Schlenk tube was placed 0.6 g of P(VDF-TrFE-CTFE) terpolymer with molar composition 61.7 / 28.3 / 10, followed by 10 mL of acetone. The mixture was stirred until the polymer dissolved. In a second Schlenk tube, 4-hydroxybenzophenone (0.79 g, 4.0 mmol), potassium carbonate (0.83 g, 6.0 mmol) and 15 mL of acetone were stirred at 50° C. for 1 hour under an inert atmosphere. After cooling the second solution to room temperature, the contents of the (second) Schlenk tube were filtered through a 1 μm PTFE filter and transferred into the first Schlenk tube, and the first Schlenk tube was heated at 50 °C 3 days. The solution was then cooled and precipitated twice from water acidified with a few drops of hydrochloric acid. The fleecy white solid was then washed twice with ethanol and twice with chloroform. The modified polymer was dried overnight at 60°C in a vacuum oven.
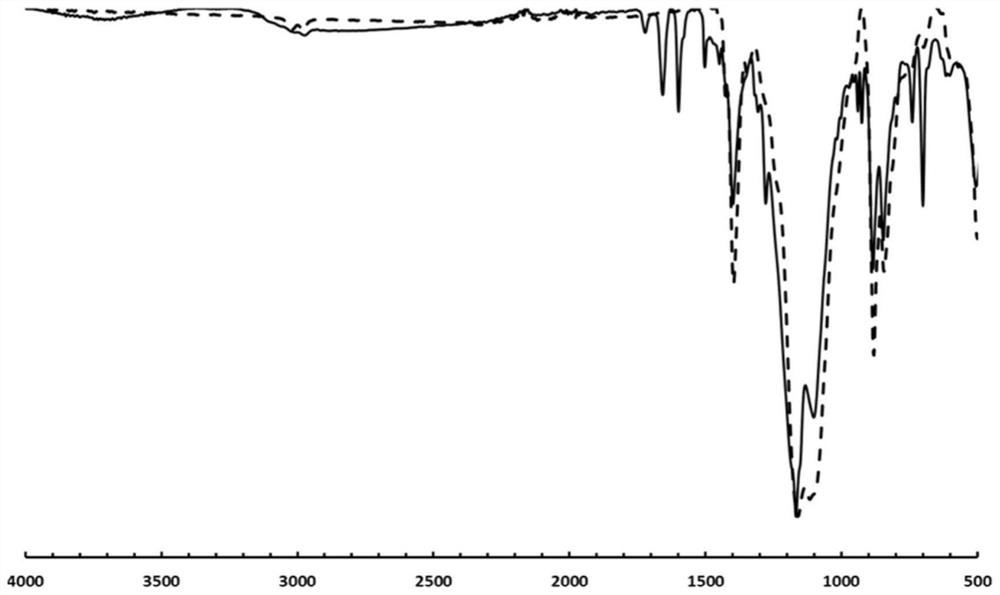
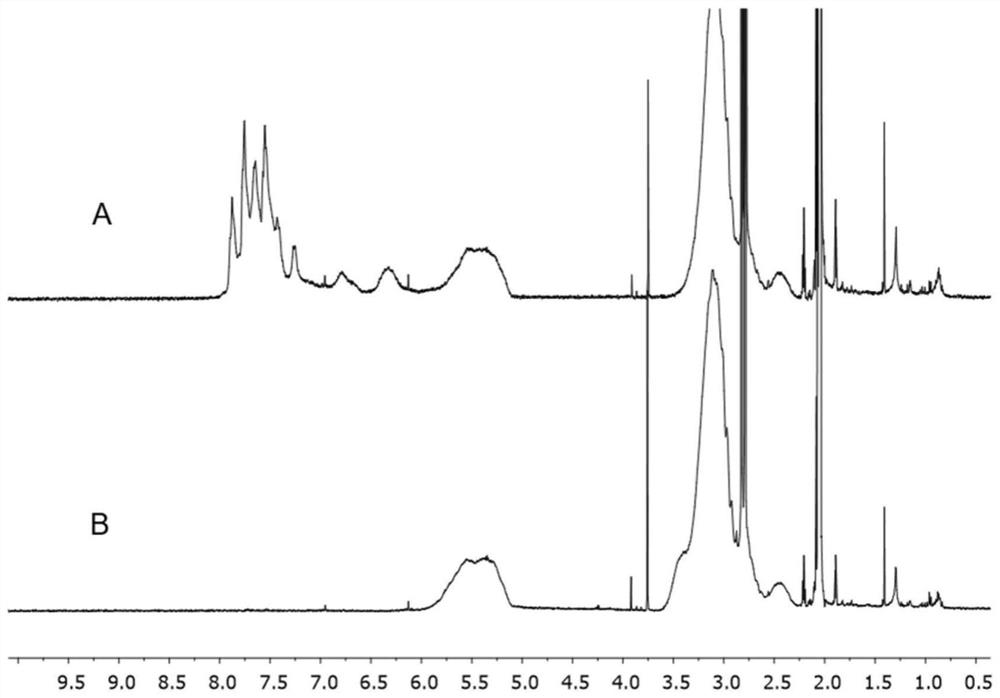
[0249] Final product by FTIR, SEC and liquid 1 H NMR characterization. The final polymer co...
Embodiment 2
[0255] A solution of the polymer synthesized as above was prepared in cyclopentanone having a proportion of 4% by mass. Films of this polymer were formed by spin coating at 1000 rpm. The polymer was subjected to heat pretreatment at 130° C. for 5 minutes. It was then placed under an inert atmosphere (nitrogen) using a mask at 6J.cm -2 doses exposed to UV radiation. The polymer irradiated selectively in a pattern was subjected to a second thermal pretreatment at 130° C. for 5 minutes. It was then developed at room temperature in a mixture of 80 / 20 mass ratio of isopropanol and cyclopentanone for 2 minutes, followed by rinsing with isopropanol.
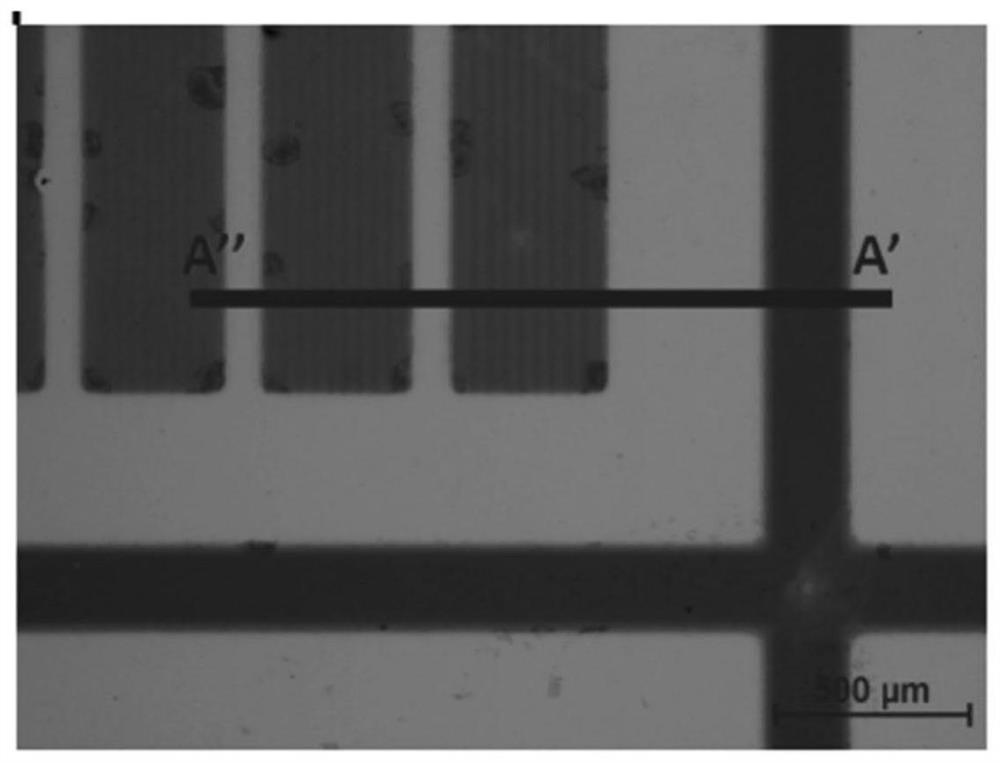
[0256] The obtained film was photographed by light microscopy (see image 3 ). The polymers correspond to darker regions.
[0257] Figure 4 is the relative dielectric permittivity curve at 1kHz. The crosslinked films retained good electroactive properties, with a relative dielectric permittivity greater than 20 and a maximum va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com