Nootkatone thioether derivative containing 1,3,4-oxadiazole ring as well as preparation method and application of notkatone thioether derivative
A technology of nokadone sulfide and oxadiazole ring, applied in the preparation of natural product pesticides, nokadone derivatives, nokadone sulfide derivatives and their preparation fields, can solve the problem of insecticide The problems of narrow insecticidal effect, less research on the synthesis of nokadone, and general activity have achieved the effects of low cost, good insecticidal activity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 contains the synthesis of noka ketone thioether derivatives of 1,3,4-oxadiazole ring
[0036] (1) Synthesis of 13-chloronokadone
[0037] Weigh nokadone (1mmol) in a 50mL round-bottomed flask, add 5mL of anhydrous dichloromethane to dissolve it, then add NCS (1.5mmol), heat and stir at 40°C, and detect with thin layer chromatography (TLC). After completion, the mixed solution was evaporated under reduced pressure, 20 mL of distilled water was added, extracted three times with ethyl acetate (30 mL), the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain 13-chloronokatone .
[0038] The physical and chemical properties of 13-chloronokadone:
[0039] 1), yellow liquid.
[0040] 2), the infrared spectrogram (IR) feature of 13-chloronokatone:
[0041] Using potassium bromide tablet method: 2937cm -1 Stretching vibration absorption for saturated hydrocarbons...
Embodiment 2
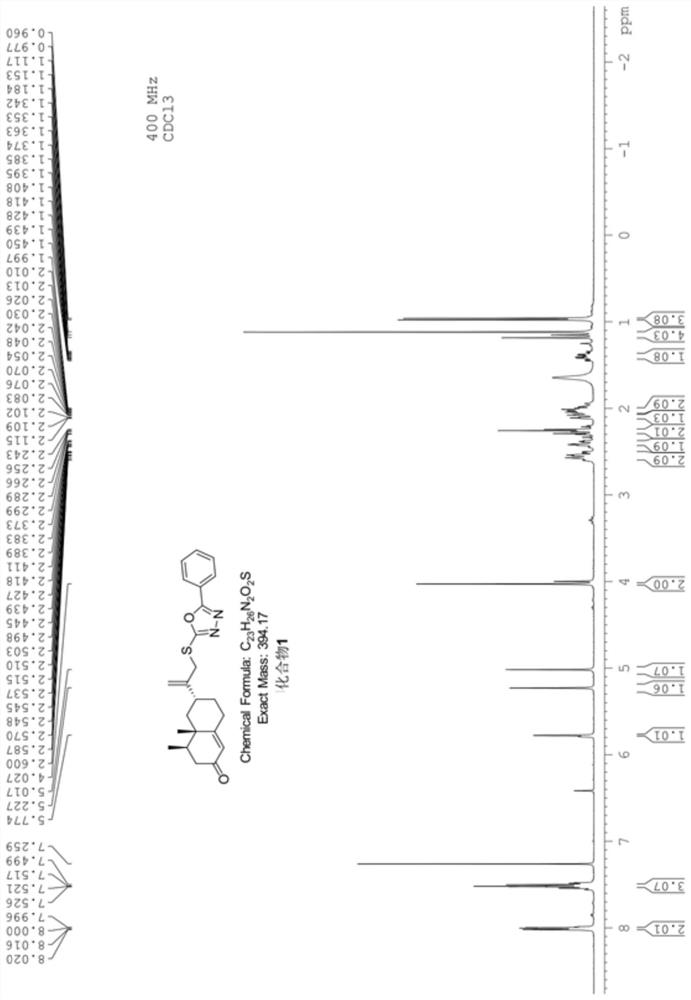
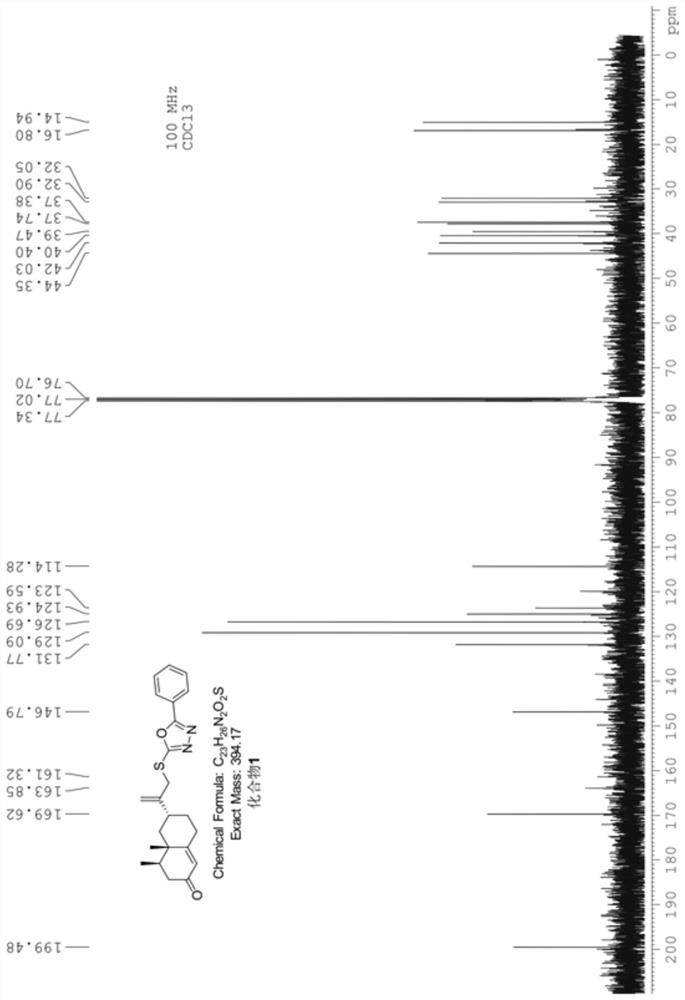
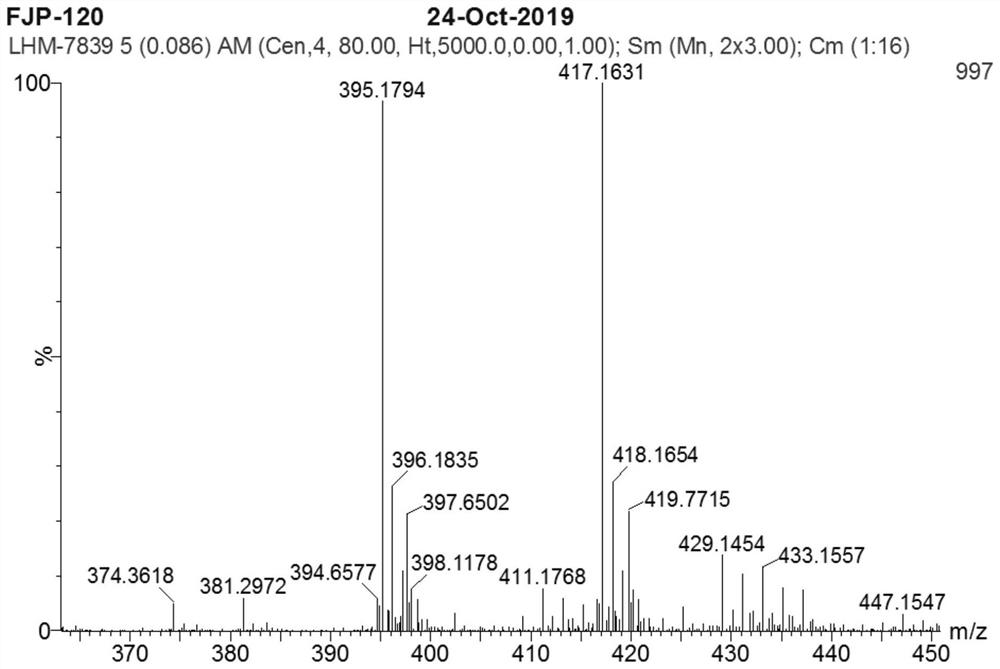
[0055] Example 2 Synthesis of Nokadone Sulfide Derivatives Containing 1,3,4-Oxadiazole Ring (Compound 2)
[0056] Using the method described in Example 1, compound 2 was synthesized. The structure and physicochemical properties of compound 2 are as follows:
[0057]
[0058] 1), yellow liquid.
[0059] 2), the infrared spectrogram (IR) feature of this compound:
[0060] Using potassium bromide tablet method: 2933cm -1 Stretching vibration absorption for saturated hydrocarbons, 1665cm -1 It is the stretching vibration of ketone carbonyl, 1473cm -1 It is saturated hydrocarbon deformation vibration, 1178, 1068cm -1 are the stretching vibrations of C-O and C-S.
[0061] 3), the nuclear magnetic resonance spectrum of this compound ( 1 HNMR, 400MHz) Features:
[0062] With deuterated chloroform as solvent, TMS as internal standard, wherein each peak belongs to: 1 H NMR (400MHz CDCl 3 )δ: 7.88(d, J=8.4Hz, 2H, -Ar), 7.29(d, J=8.0Hz, 2H, -Ar), 5.77(s, 1H, H-1), 5.21(s, 1H, ...
Embodiment 3
[0065] Example 3 Synthesis of Nokadone Sulfide Derivatives Containing 1,3,4-Oxadiazole Ring (Compound 3)
[0066] Using the method described in Example 1, compound 3 was synthesized. The structure and physicochemical properties of compound 3 are as follows:
[0067]
[0068] 1), white solid, melting point 92-93°C.
[0069] 2), the infrared spectrogram (IR) feature of this compound:
[0070] Using potassium bromide tablet method: 2934cm -1 Stretching vibration absorption for saturated hydrocarbons, 1675cm -1 It is the stretching vibration of ketone carbonyl, 1465cm -1 It is saturated hydrocarbon deformation vibration, 1193, 1071cm -1 are the stretching vibrations of C-O and C-S.
[0071] 3), the nuclear magnetic resonance spectrum of this compound ( 1 HNMR, 400MHz) Features:
[0072] With deuterated chloroform as solvent, TMS as internal standard, wherein each peak belongs to: 1 H NMR (400MHz CDCl 3 )δ:7.86-7.88(m,2H,-Ar),7.63-7.65(m,2H,-Ar),5.77(s,1H,H-1),5.22(s,1H,H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com