Preparation method of mussel-imitating hydrogel catalytically crosslinked by hemoglobin
A hemoglobin and hydrogel technology, which is applied in the field of biocatalysis, can solve the problems of gel biocompatibility or adverse effect on strength, high price, unsuitable for large-scale production and application of hydrogel, etc., and achieves good antibacterial and hemostatic effects. , low cost, good biocompatibility and degradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of CHI-C Hydrogel Precursor
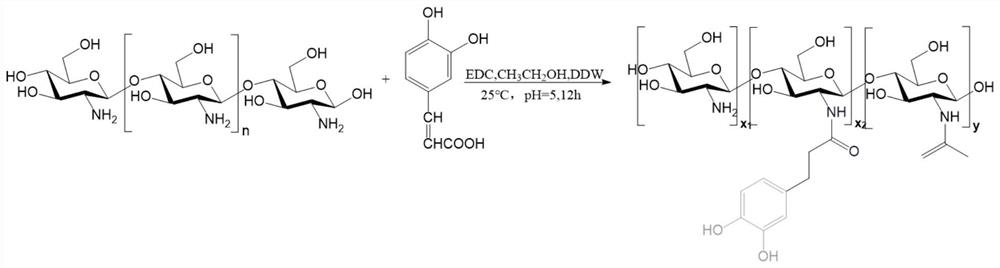
[0030] The preparation process of the hydrogel precursor (chitosan-caffeic acid derivative): 3g chitosan was dissolved in 100ml of hydrochloric acid solution with a pH value of 5; 2.37g of caffeic acid and 2.02g of EDC were dissolved in 25ml of deionized water; Acid and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) solution were slowly added to the chitosan solution; in order to prevent catechol from being oxidized, the pH of the solution was kept at 5.0. Add 50ml of ethanol to the mixture as a co-solvent and stir at room temperature to avoid possible precipitation in the EDC reaction; after 12 hours, dialyze in a pH 4.0 sodium chloride solution for 2 days, then dialyze in deionized water for 4h, and purify The product chitosan-caffeic acid derivative (CHI-C) is obtained; finally the product is freeze-dried and stored in a moisture-free desiccator before use. Reactions such as figure 1 .
[0031] 2. Prep...
Embodiment 2
[0034] 1. Preparation of P(MEO 2 MA-OEGMA-DMA) hydrogel precursor
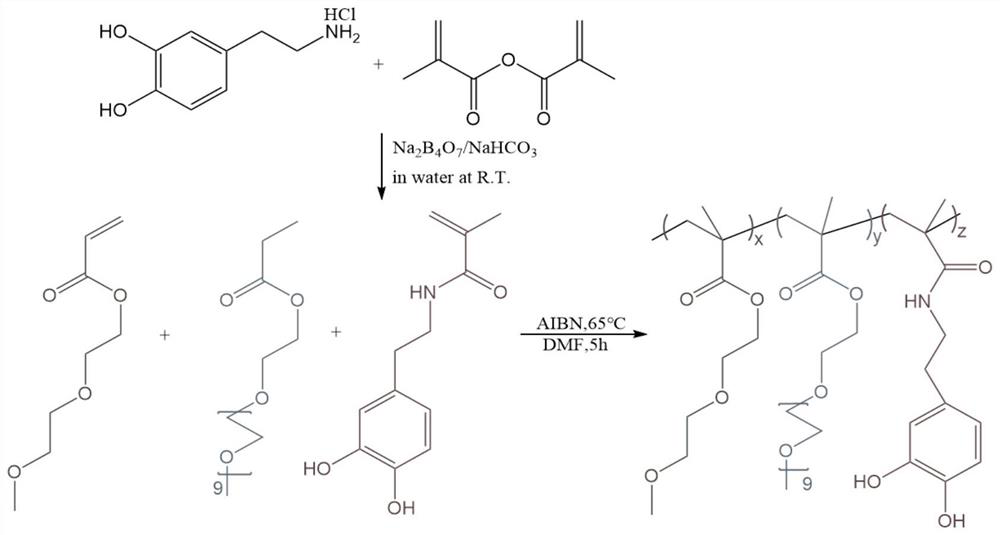
[0035] 8g sodium bicarbonate (NaHCO 3 ) and 20g sodium tetraborate decahydrate (Na 2 B 4 o 7 10H 2 O) Dissolve in 200mL of double distilled water, and insert the solution with a needle, nitrogen bubbles for 30 minutes to remove oxygen in the solution; subsequently, add 10g of dopamine hydrochloride in the solution; dissolve 9.4mL of methacrylic anhydride in 50mL of tetrahydrofuran And use a constant pressure funnel to add dropwise to the mixed solution, adjust the pH of the mixed solution to >8 with 1M sodium hydroxide; stir and react at room temperature for 16 hours, and filter the reacted mixed solution under reduced pressure to remove NaHCO 3 and Na 2 B 4 o 7 10H 2 O solid; the clear and transparent filtrate was washed with 100 mL of ethyl acetate, and the pH was adjusted to 4 ) sealed in the refrigerator and refrigerated and dried overnight; the dried solution was filtered to remove MgSO 4 , conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com