Novel method for preparing sodium bicarbonate and co-producing ammonium sulfate from sodium sulfate
A technology of sodium bicarbonate and ammonium bicarbonate, applied in the field of chemical technology, can solve the problems of waste of resources, low overall yield, less than 60% utilization rate of sodium sulfate, etc., and achieve the effect of clean process and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
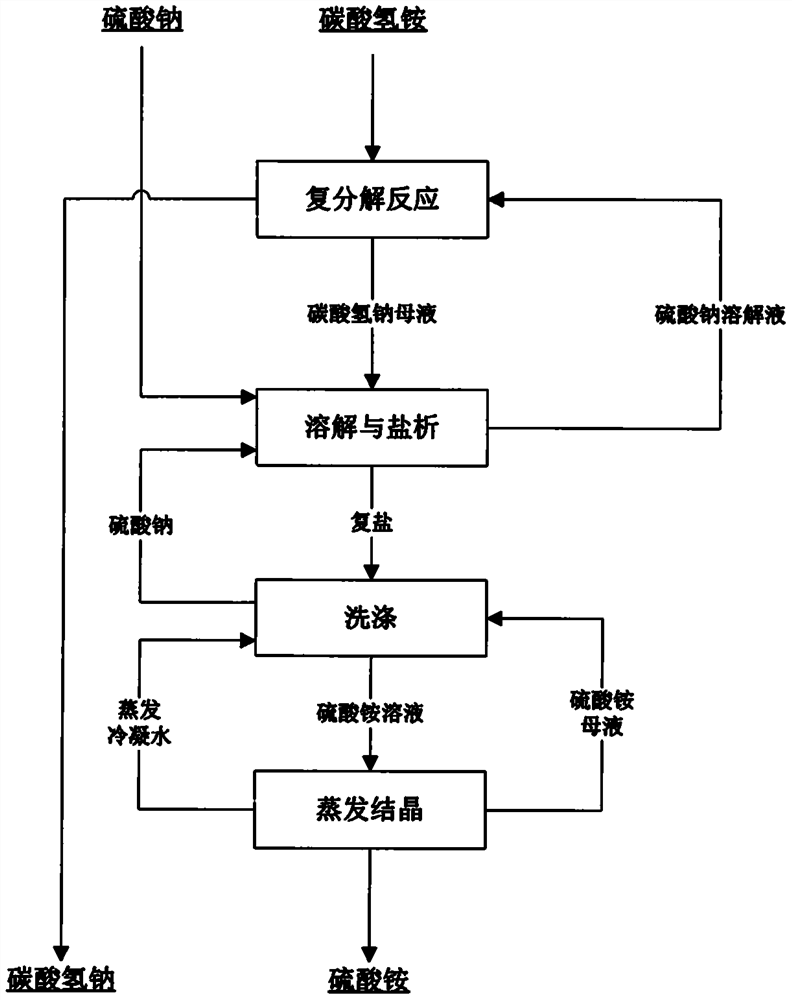
[0033] A kind of novel method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0034] (1) Sodium sulfate is dissolved in water to obtain a sodium sulfate solution. At 25°C, ammonium bicarbonate is slowly added. The molar ratio of ammonium to sodium in the solution is 1.3:1; after 5 hours of reaction, after liquid-solid separation Obtain sodium bicarbonate solid and sodium bicarbonate mother liquor;
[0035] (2) in the sodium bicarbonate mother liquor that step (1) obtains, add sodium sulfate (comprising the sodium sulfate that the outside adds and the sodium sulfate two parts that step (3) obtains), the total amount that sodium sulfate adds is in the sodium bicarbonate mother liquor 1.4 times the mass of total sodium sulfate, react at 90°C for 1 hour, then cool down to 40°C to salt out ammonium sulfate, and obtain double salt and sodium sulfate solution composed of sodium sulfate and ammonium sulfate respectively after liqu...
Embodiment 2
[0040] A kind of novel method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0041] (1) To the sodium sulfate solution obtained in step (2), slowly add ammonium bicarbonate at 50°C, the molar ratio of ammonium and sodium in the solution is 1:1; after reacting for 1 hour, after liquid-solid separation Obtain sodium bicarbonate solid and sodium bicarbonate mother liquor;
[0042] (2) in the sodium bicarbonate mother liquor that step (1) obtains, add sodium sulfate (comprising the sodium sulfate that the outside adds and the sodium sulfate two parts that step (3) obtains), the total amount that sodium sulfate adds is in the sodium bicarbonate mother liquor 1.1 times the mass of total sodium sulfate, react at 50°C for 4 hours, then cool down to 10°C to salt out ammonium sulfate, and obtain double salt and sodium sulfate solution composed of sodium sulfate and ammonium sulfate respectively after liquid-solid separation;
[0043...
Embodiment 3
[0047] A kind of novel method that sodium sulfate prepares sodium bicarbonate to co-produce ammonium sulfate, comprises the steps:
[0048] (1) To the sodium sulfate solution obtained in step (2), slowly add ammonium bicarbonate at 35°C, the molar ratio of ammonium and sodium in the solution is 1.1:1; after reacting for 3 hours, after liquid-solid separation Obtain sodium bicarbonate solid and sodium bicarbonate mother liquor;
[0049] (2) add sodium sulfate to the sodium bicarbonate mother liquor that step (1) obtains and salt out ammonium sulfate simultaneously (comprising the sodium sulfate that the outside world adds and the sodium sulfate two parts that step (3) obtains), the total amount that sodium sulfate adds is 1.2 times the mass of total sodium sulfate in the mother liquor of sodium bicarbonate, react at 60°C for 1.5h, then cool down to 28°C to salt out ammonium sulfate, and obtain double salt composed of sodium sulfate and ammonium sulfate and sodium sulfate after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com