Polyaspartate, and preparation method and application thereof
A technology of aspartic acid ester and maleic acid diol ester, which is applied in the field of polymer synthesis, can solve the problems of high esterification temperature, poor coating performance, short construction operation time, etc., and achieve high esterification conversion rate , Conducive to construction penetration, improve the effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
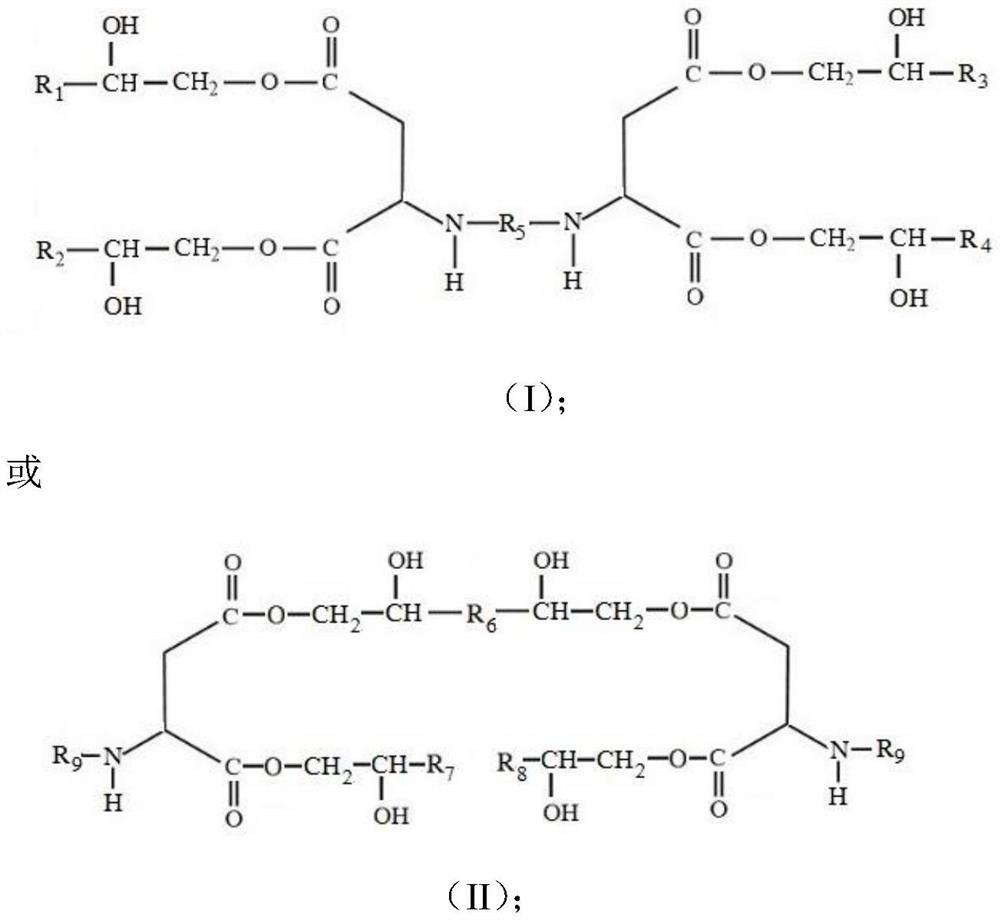
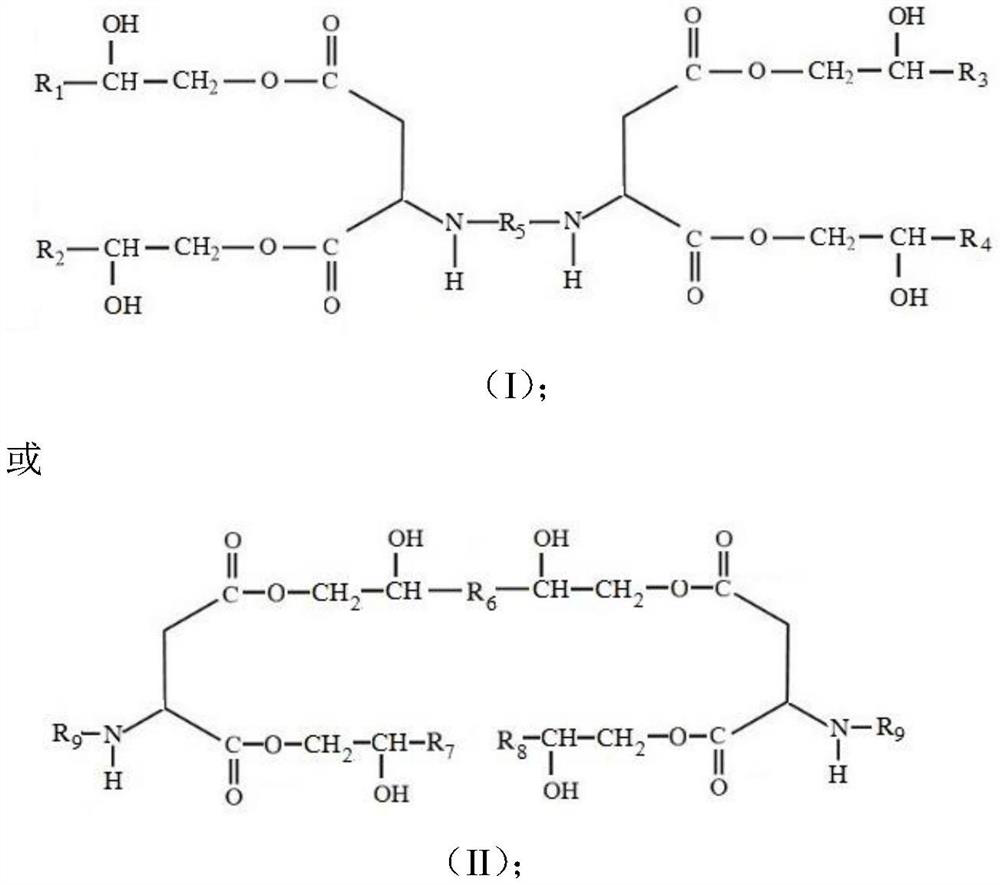
[0040] A kind of polyaspartic acid ester, structural formula is:
[0041]
[0042] Among them, R 1 , R 2 , R 3 and R 4 is methyl; R 5 for polyether groups.
[0043] Its preparation method comprises the following steps:
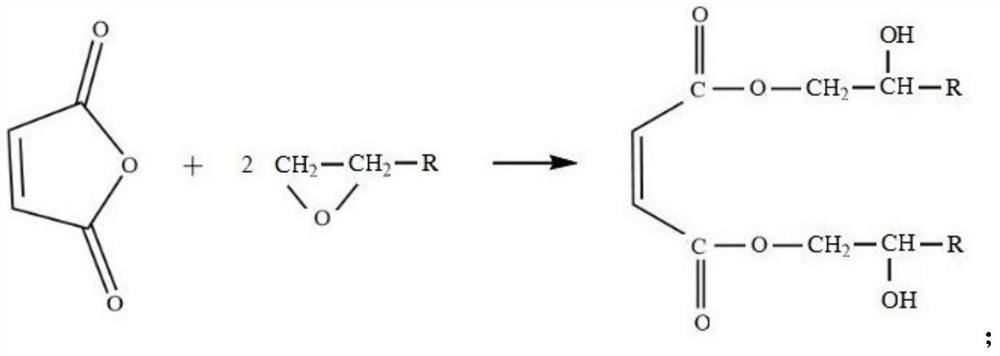
[0044] (1) Add maleic anhydride, polymerization inhibitor p-hydroxyanisole (MEHQ), catalyst triphenylphosphine into the flask, drop propylene oxide, the mol ratio of maleic anhydride and propylene oxide is 1:2.1, The addition amount of MEHQ is 0.02% of the total mass of the reactants, and the addition amount of triphenylphosphine is 0.02% of the total mass of the reactants; the addition rate of propylene oxide is controlled so that the reaction temperature does not exceed 60 ° C, and the addition is completed at 70 ° C Insulate for 2 hours, measure the acid value every 0.5 hours, and wait until the acid value reaches 2mgKOH / g to discharge to obtain diol maleate;
[0045] (2) Add polyetheramine D400 to the flask, and add sodium methoxide, the amount o...
Embodiment 2
[0047] A kind of polyaspartic acid ester, structural formula is:
[0048]
[0049] Among them, R 1 and R 2 for hydrogen, R 3 and R 4 is methyl; R 5 For n-hexyl.
[0050] Its preparation method comprises the following steps:
[0051] (1) Add maleic anhydride, polymerization inhibitor p-hydroxyanisole (MEHQ), and catalyst triphenylphosphine into the flask, add dropwise a mixture of ethylene oxide and propylene oxide, maleic anhydride, ethylene oxide The mol ratio with propylene oxide is 1:1:1, and the addition amount of MEHQ is 0.02% of reactant gross mass, and the addition amount of triphenylphosphine is 0.01% of reactant gross mass; The drop rate of oxypropane is such that the reaction temperature does not exceed 60°C. After the dropwise addition, keep the temperature at 60°C for 6h, and measure the acid value every 0.5h. When the acid value reaches 2mgKOH / g, the material is discharged to obtain diol maleate;
[0052] (2) Add 1,6-hexamethylenediamine into the flask, ...
Embodiment 3
[0054] A kind of polyaspartic acid ester, structural formula is:
[0055]
[0056] Among them, R 1 , R, R 3 and R 4 Respectively hydrogen, methyl, ethyl and cyclopentyl; R 5 For cycloalkyl.
[0057] Its preparation method comprises the following steps:
[0058] (1) Add maleic anhydride, polymerization inhibitor p-hydroxyanisole (MEHQ), and catalyst triphenylphosphine into the flask, and drop propylene oxide and ethylene oxide with a molar ratio of 1:1:1:1 , a mixture of butylene oxide and 1,2-epoxycyclopentane, the molar ratio of maleic anhydride and epoxy groups in the mixture is 1:2.4, the amount of MEHQ added is 0.02% of the total mass of reactants, triphenyl The addition amount of base phosphine is 0.1% of the total mass of reactants; control the dropping speed of the epoxy mixture so that the reaction temperature does not exceed 60°C, keep the temperature at 70°C for 2h after dropping, measure the acid value every 0.5h, and wait until the acid value reaches 2mgKO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com