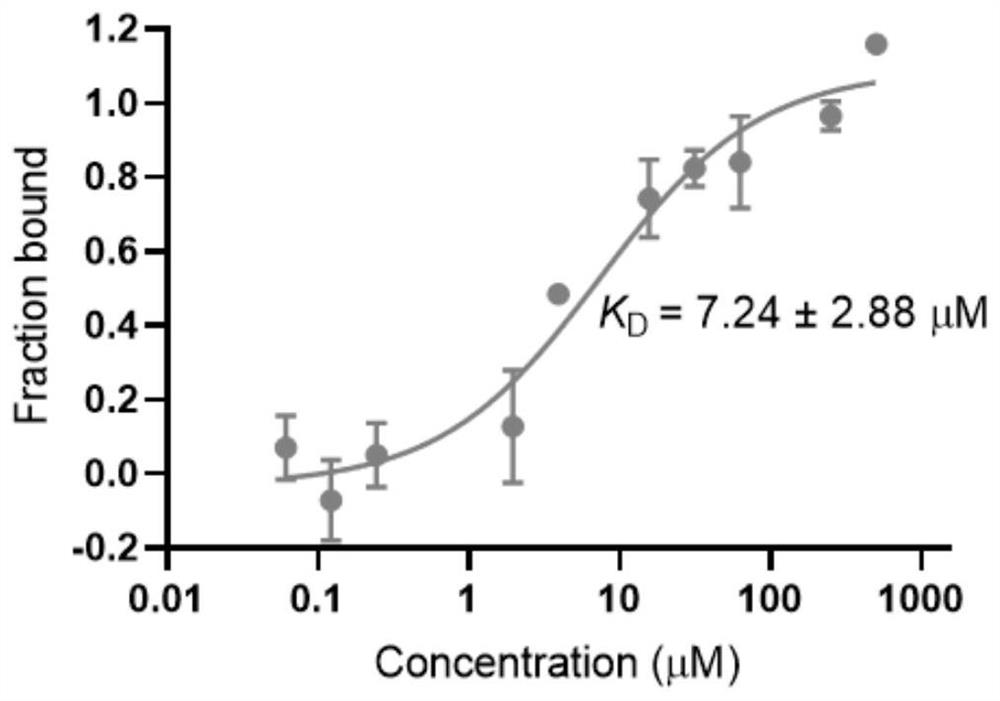
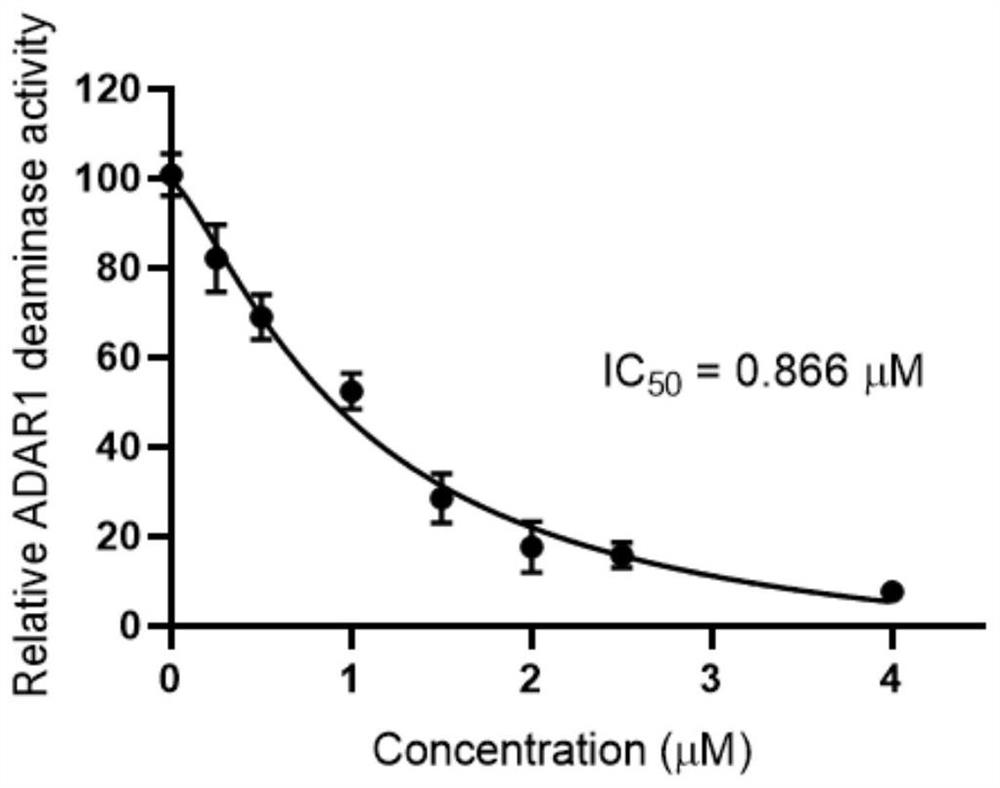
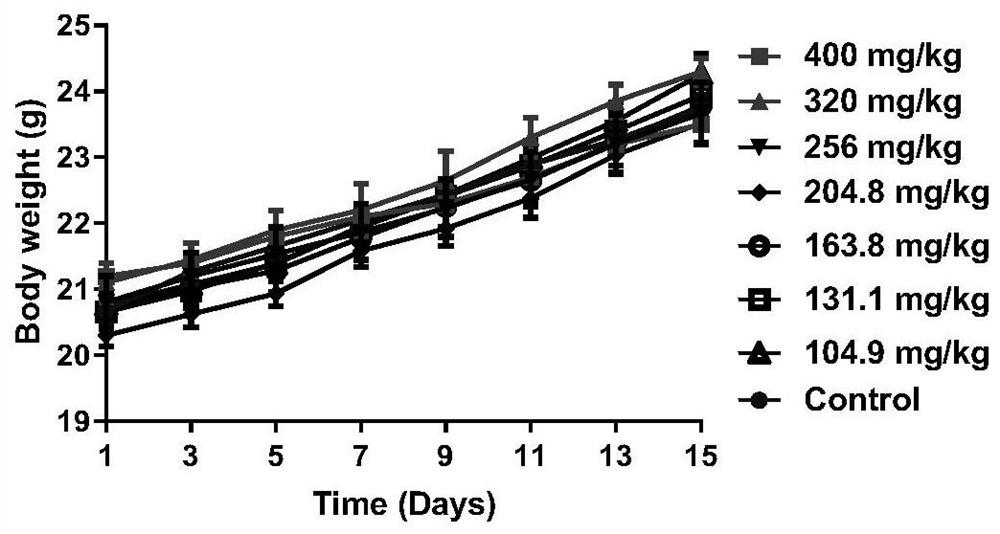
Polysubstituted purine compound as well as preparation method and application thereof
A compound and purine technology, applied in the field of polysubstituted purine compounds and their preparation, to achieve the effects of low toxicity, inhibition of cancer proliferation and metastasis, and resistance to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: (2R, 3R, 4S, 5S)-2-(6-amino-2-fluoro-9H-purin-9-yl)-5-(chloromethyl)tetrahydrofuran-3,4-diol ( I-1) synthesis:
[0077]
[0078] At 0°C, 2-fluoroadenosine A-1 (5.70g, 20mmol) was dissolved in acetonitrile (80mL), pyridine (3.22mL, 40mmol) was added, and thionyl chloride (7.25mL , 100mmol), stirred for 4h and raised to room temperature, reacted overnight, TLC monitored that the raw materials were completely consumed, and the solvent was removed by rotary evaporation under reduced pressure, and methanol (120mL), water (12mL), and ammonia water (24mL) were added again, stirred for 0.5h, and reduced pressure Concentrated by rotary evaporation, the product was precipitated in the water phase, filtered to obtain a filter cake, redissolved in a small amount of methanol at 60°C, added dropwise with dichloromethane, cooled to precipitate a solid and suction filtered to obtain a filter cake, washed with cold methanol to obtain the product I-1 as a white solid (5.45 ...
Embodiment 2
[0079] Example 2: 9-((3aR,4R,6R,6aR)-6-(((tert-butyldimethylsilyl)oxy)methyl)-2,2-dimethyltetrahydrofuran[3, Synthesis of 4-d][1,3]bisoxazol-4-yl)-2-fluoro-9H-purin-6-amine (I-2):
[0080]
[0081] Step 1, 2-fluoroadenosine (1.48g, 5mmol) was dissolved in anhydrous acetone (200mL) to form a suspension, anhydrous p-toluenesulfonic acid (4.31g, 25mmol) was added to form a clear solution, and dimethyl Oxypropane (1.04g, 10mmol), after the mixture was stirred at room temperature under nitrogen atmosphere for 4h, cold saturated NaHCO 3 The solution (100 mL) was added to the above mixture; the volatiles were removed under reduced pressure and the residue was dried; the resulting solid was dissolved in acetone (400 mL), stirred for 1 h, then filtered; the volatiles were removed and the crude product was subjected to column chromatography Purified by method to obtain white solid A2 (1.50g, 95%); 1 H NMR (400MHz, DMSO-d 6 )δ8.33(s,1H),7.92(s,2H),6.03(d,J=2.9Hz,1H),5.29(dd,J=6.2,2...
Embodiment 3
[0083] Example 3: ((3aR,4R,6R,6aR)-6-(6-amino-2-fluoro-9H-purin-9-yl)-2,2-dimethyltetrahydrofuran[3,4-d] Synthesis of [1,3]bisoxazol-4-yl)methyl acetate (I-3):
[0084] A2 (0.66g, 2mmol) was dissolved in 10mL DMF, triethylamine (0.83mL, 6mmol), acetic anhydride (0.21mL, 2.2mmol) and DMAP (0.05g, 0.4mmol) were added and reacted at room temperature overnight. TLC monitoring raw material consumption is complete, saturated NH 4 Cl aqueous solution (5 mL) quenched the reaction, the mixture was extracted with ethyl acetate (3 × 30 mL), the combined organic layers were washed with saturated aqueous NaCl solution, anhydrous NaCl 2 SO 4 It was dried, filtered and concentrated under reduced pressure, and purified by silica gel column chromatography to obtain compound I-3 (0.66 g, 90%). 1 HNMR (400MHz, DMSO-d 6 )δ8.28(s,1H),7.92(d,J=26.2Hz,2H),6.11(d,J=2.4Hz,1H),5.41(dd,J=6.2,2.5Hz,1H),5.00( dd,J=6.2,3.3Hz,1H),4.45–4.30(m,1H),4.30–4.08(m,2H),1.96(s,3H),1.54(s,3H),1.34(s,3H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com