Codon-optimized danio rerio g-type lysozyme-1 gene and recombinant expression protein thereof
A technology of codon optimization and recombinant protein is applied in the field of zebrafish g-type lysozyme-1 gene and its recombinant expression protein, which can solve the problems of bacterial stress response ability and unknown function, and achieve the effect of high bacteriolytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Codon optimization and expression vector construction of zebrafish g-type lysozyme-1 gene
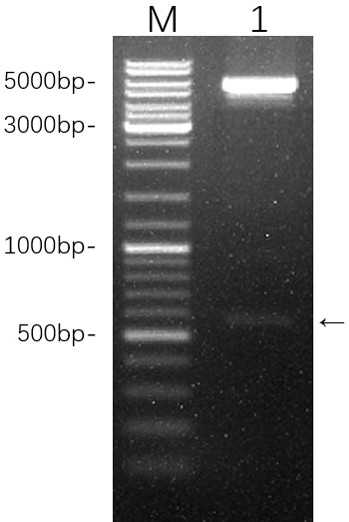
[0020] The open reading frame sequence of the zebrafish g-type lysozyme-1 gene (accession number: NM_001002706.1) was obtained from the GenBank database, as shown in SEQ ID NO.1. First, signal peptide analysis was performed on the sequence, and the signal peptide sequence ATGGGCATTCCGGTGATACTTACCATGTATTTCTAGCATGCATTTATGGA in the sequence of SEQ ID NO.1 was removed. According to the codon preference of Escherichia coli and the principle of avoiding repetitive sequences, the codon optimization software was used to optimize the codon of the gene sequence without changing the amino acid sequence ( figure 1 ), and joined at the 5' end of the sequence Bam HI restriction site, added at the 3' end Xho I restriction site. The codon-optimized zebrafish g-type lysozyme-1 gene nucleotide sequence is shown in SEQ ID NO.2, and its encoded amino acid sequence is shown in SEQ ID ...
Embodiment 2
[0036] Example 2 Expression and purification of zebrafish g-type lysozyme-1 gene
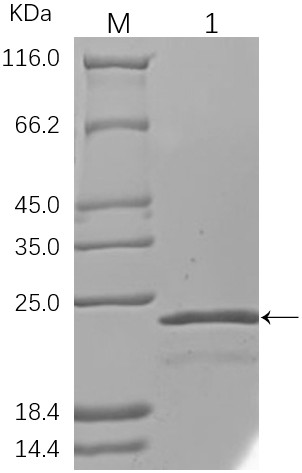
[0037] The recombinant plasmid 28a-Lys-g1 was transformed into BL21 (DE3) Competent cells, pick a single colony and culture in LB liquid medium containing 50 μg / ml kanamycin, when the OD 600 When the value reached 0.6, IPTG was added to a final concentration of 0.5 mM, and cultured at 20°C for 16 h. The cells were collected by centrifugation, resuspended in imidazole buffer, disrupted by ultrasonication, and the supernatant was collected. Take 5 mL of Ni-NTA and wash the equilibrated column with 5 times the bed volume of Binding buffer at a flow rate of 5 mL / min. The supernatant was incubated with the equilibrated column packing for 1 h, and then loaded onto the column. The equilibrated column was first washed with urea buffer, then washed with urea-containing imidazole buffer and eluted, and the effluent was collected. The purified protein was dialyzed and concentrated, filtered through a ...
Embodiment 3
[0038] Example 3 Determination of zebrafish g-type lysozyme-1 recombinantly expressed protein concentration and activity detection
[0039] The concentration of purified zebrafish g-type lysozyme-1 recombinant protein was determined by a non-interfering protein concentration assay kit (Sangon Bioengineering (Shanghai) Co., Ltd.). First, take 12 1.5 mL centrifuge tubes, each with the same number, and add 0, 4, 8, 12, 20, 25 μL of BSA standard protein solution (2 mg / mL) to the centrifuge tubes with different numbers, respectively. Add the same volume of protein standard solution to the same numbered centrifuge tube. Then take two 1.5 mL centrifuge tubes and add 10 μL of recombinant protein solution to each. Add 0.5 mL of Precipitation Reagent 1 to each of the above 14 test tubes, vortex for 30 sec, and place at room temperature for 2-3 min. Then add 0.5 mL of precipitation reagent 2 separately, and vortex for 30 sec. Transfer to a refrigerated centrifuge and centrifuge at 13,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com