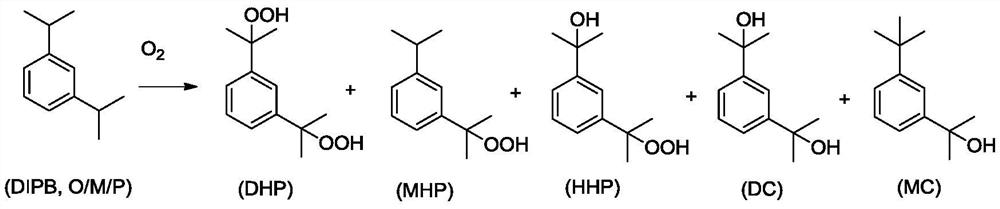
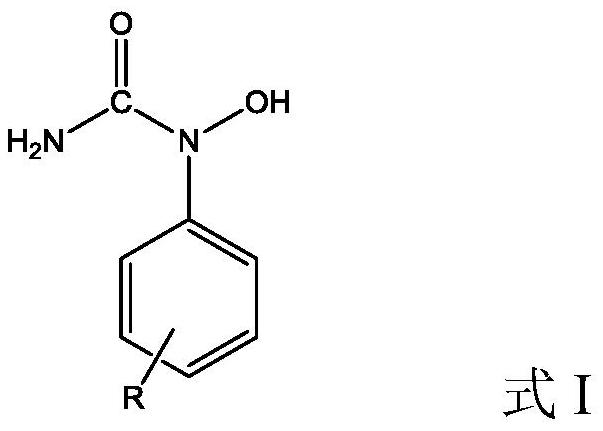
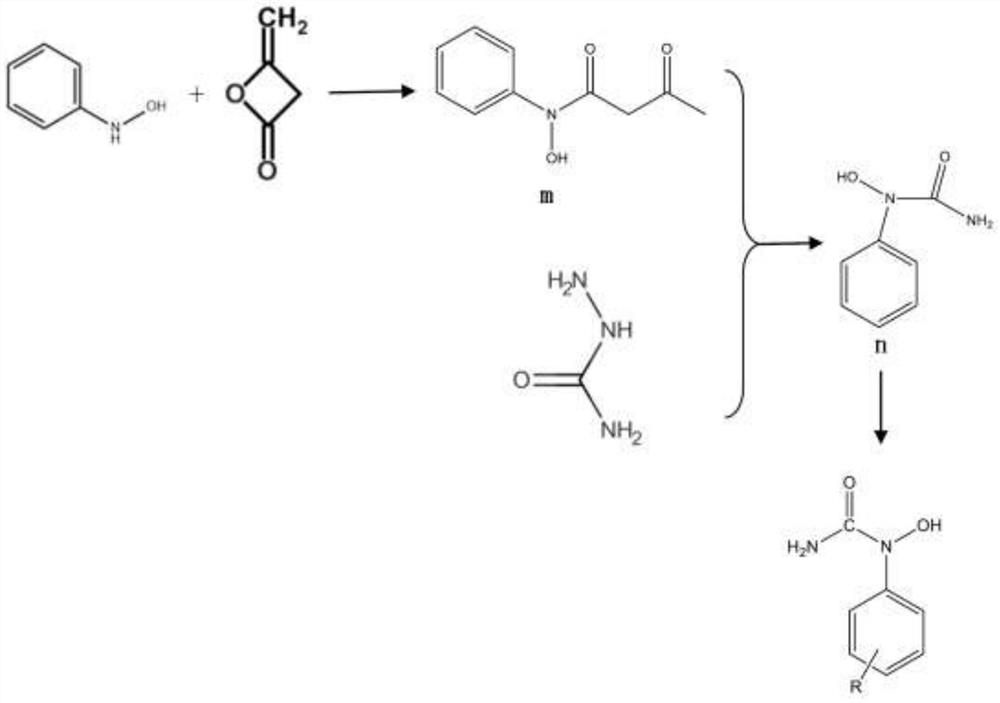
A kind of preparation method of dicumyl hydroperoxide
A technology of dicumyl hydroperoxide and propylbenzene hydroperoxide, which is applied in the direction of the preparation of peroxy compounds, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problem of decomposition of dicumyl hydroperoxide , Unable to realize continuous production, incompatibility of inorganic alkali solution and other problems, to achieve the effect of simple preparation method, easy continuous production, and promotion of neutralization reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] [Preparation Example 1] Preparation of Catalyst A
[0057](1) 0.2 mol of phenylhydroxylamine and 0.06 mol of diketene were dissolved in 400 ml of carbon tetrachloride solvent, stirred, heated to 80° C., refluxed for 10 h, and the reaction solution was separated by silica gel column chromatography to obtain intermediate m, The yield is about 40%.
[0058] Silica gel column chromatography conditions: the solvent was removed by rotary evaporation, and the crude product was separated and purified by column chromatography after concentration (eluent: ethyl acetate: petroleum ether=9:1).
[0059] (2) Dissolve 0.2 mol of intermediate m and 0.4 mol of semicarbazide in 500 ml of ethanol, stir, heat to 80° C., reflux for 8 h, and separate the reaction solution by silica gel column chromatography to obtain intermediate n with a yield of about 20% .
[0060] Silica gel column chromatography conditions: the solvent was removed by rotary evaporation, and the crude product was separ...
Embodiment 2
[0067] Using the intermediate n prepared in Preparation Example 1 as a raw material, prepare catalyst B:
[0068] 0.2 mol of intermediate n, 0.2 mol of propylene, 10 g of ZSM-5 molecular sieve catalyst, 200 ml of methanol solvent, 100° C. and 1 MPa pressure were stirred and reacted for 2 h, and catalyst B was obtained by separation.
[0069] The NMR analysis data are as follows:
[0070] 1 H NMR (500MHz, CDCL3): δ 7.77–7.38 (m, 2H), 7.28–6.90 (m, 2H), 4.92 (s, 2H), 3.23 (s, 1H), 2.87 (m, 1H), 1.20 (d, J=12.8Hz, 6H).
[0071] 13 C NMR: (125MHz, CDCL3) δ162.27, 147.64, 147.35, 126.27, 121.68, 33.96, 23.38.
[0072] The structure of analytical catalyst B is represented as follows:
[0073]
Embodiment 3
[0075] Using the intermediate n prepared in Preparation Example 1 as a raw material, prepare catalyst C:
[0076] 0.2 mol of intermediate n, 0.2 mol of 1-heptene, 10 g of ZSM-5 molecular sieve catalyst, 200 ml of methanol solvent, 130° C. and 0.3 MPa pressure were stirred and reacted for 2 h, and catalyst C was obtained by separation.
[0077] The NMR analysis data are as follows:
[0078] 1 H NMR (500MHz, CDCL3): δ7.61(s,1H), 7.58–7.48(m,2H), 7.11–7.01(m,2H), 5.68(s,2H), 2.67–2.50(m,1H) ,1.68–1.52(m,2H),1.34–1.20(m,6H),1.18–1.10(d,J=13.0Hz,3H),0.93–0.85(m,3H).
[0079] 13 C NMR (125MHz, CDCL3): δ162.27, 146.02, 144.52, 126.42, 121.88, 39.67, 36.08, 31.59, 27.65, 23.16, 20.62, 14.00.
[0080] The structure of analytical catalyst C is expressed as follows:
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com