Lipopeptide based on melittin and preparation method and application thereof
A technology of melittin and lipopeptide, applied in the preparation method of peptide, botany equipment and method, application, etc., can solve the problems of high hemolysis and low hemolysis, and achieve the effect of reducing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
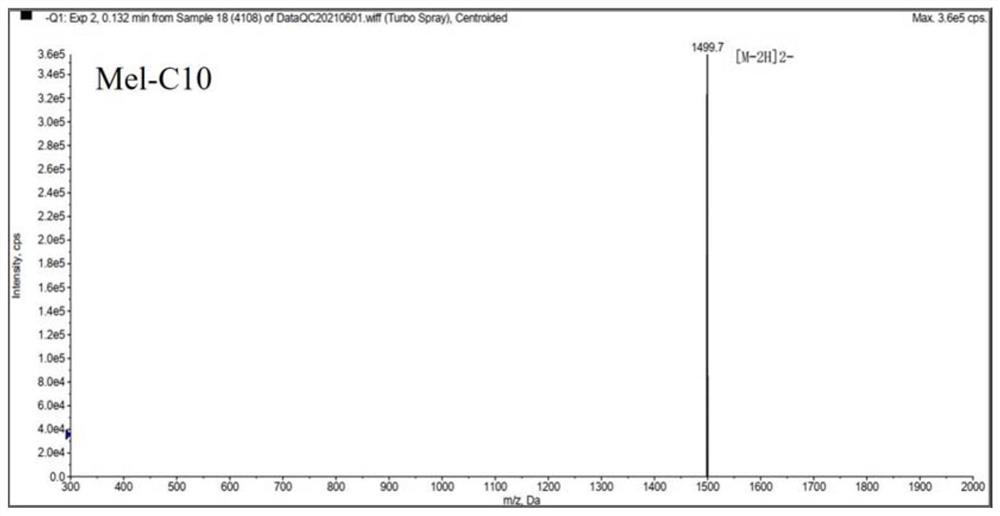
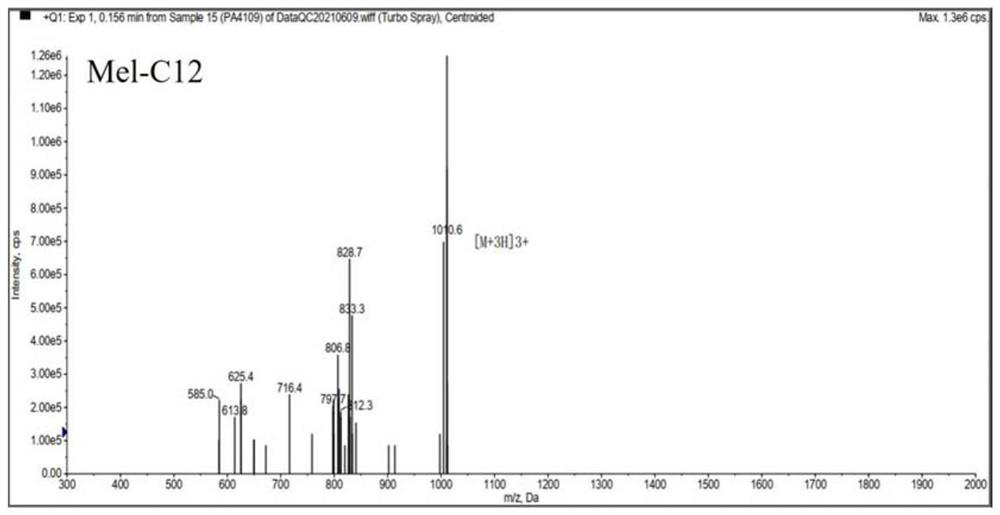
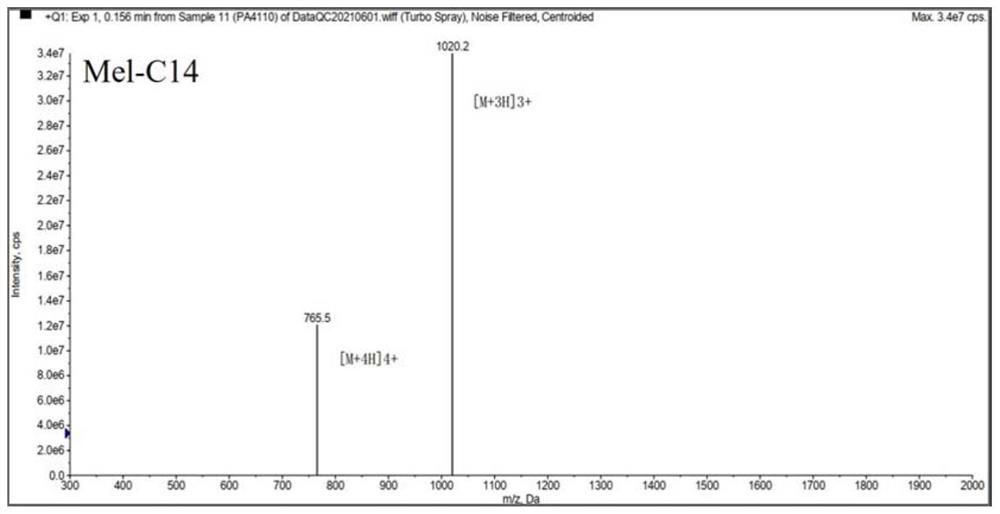
[0036] Melittin and melittin-based lipopeptides were artificially synthesized according to the melittin sequence: GIGAVLKVLTTGLPALISWIKRKRQQ-NH2.
[0037] The synthesis steps are as follows:
[0038] (1) Immobilize the carboxyl group of the first amino acid at the carbon end on 2-chloro-trityl chloride resin
[0039]Weigh 2.0g of 2-chloro-trityl chloride resin (the degree of substitution is 0.4mmol·g-1, the content of available chlorine on the resin is 2.0×0.4=0.8mmol), add it to the In the reaction column of amide (DMF), stir for 1 hour to fully swell the resin. DMF was removed, and a DMF solution dissolved in Fmoc-Gln-OH (2×0.8mmol) and diisopropylethylamine (DIEA) (4×0.8mmol) was added to the resin, and the reaction was shaken at room temperature for 2 hours. The reaction solution was removed, and the resin was washed 3 times with DMF, and then a DMF mixed solution containing excess methanol and (4×0.8 mmol) DIEA was added to continue stirring for 1 hour to block the acti...
experiment example 1
[0049] Experimental example 1: Evaluation of antibacterial activity of lipopeptide
[0050] Antibacterial assays were performed using a broth-based microdilution method in accordance with the CLSI Guidelines for Antimicrobial Susceptibility Testing. E.coli ATCC 25922, E.coli ML-35ATCC 43837, K.pneumonia ATCC 700603, P.aeruginosa FADDI-PA070, P.aeruginosa ATCC 27853, S.aureus ATCC 25923, S.aureusATCC 43300, B.subtilis ATCC, 23857 Methicillin-resistant S.aureus 936 was cultured in LB medium, NB medium or MHB medium at 37°C for overnight recovery. The recovered bacterial solution was diluted 40 times in fresh MHB medium and incubated at 37°C for 1.5-2 hours. The working solution of lipopeptide was prepared from the storage solution (vancomycin and colistin sulfate were used as antibiotic control), the concentration gradient was set to 2 times, and the concentration was from 0.015 to 64 μg / mL, in duplicate. The working solution was added to the 96-well plate, and 5 parallels wer...
experiment example 2
[0055] Experimental example 2: Hemolytic evaluation of lipopeptide
[0056] Rabbit red blood cells were separated from rabbit blood, washed with phosphate buffered saline (PBS, pH 7.4), and centrifuged at 4000 rpm for 4 to 5 times. Then, erythrocytes were dispersed in 0.25% (v / v) phosphate buffered saline solution. Prepare stock solutions of melittin and lipopeptide at 512 μM. The lipopeptide stock solution was diluted 2-fold with PBS to obtain working solutions with eight concentration gradients. Subsequently, an equal volume of red blood cell suspension and different concentrations of lipopeptide working solutions were added to the 96-well plate, and incubated at 37°C for 1 hour. After the 96-well plate is centrifuged, carefully transfer the centrifuged supernatant to a new well plate, and set it as a positive control in 490nm Triton X-100 solution (1%, v / v 20μL) to represent 100% cell hemolysis, red blood cells PBS solution (20 μL) was used as a negative control group. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com