Carbon-coated nickel oxide catalyst and preparation method and application thereof
A carbon-coated nickel and catalyst technology, applied in the field of carbon-coated nickel oxide catalyst and its preparation, can solve the problems of low activity, limiting the large-scale application of noble metal catalysts, high price, etc., and achieve improved catalytic activity and good industrial application Prospects, effects of improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
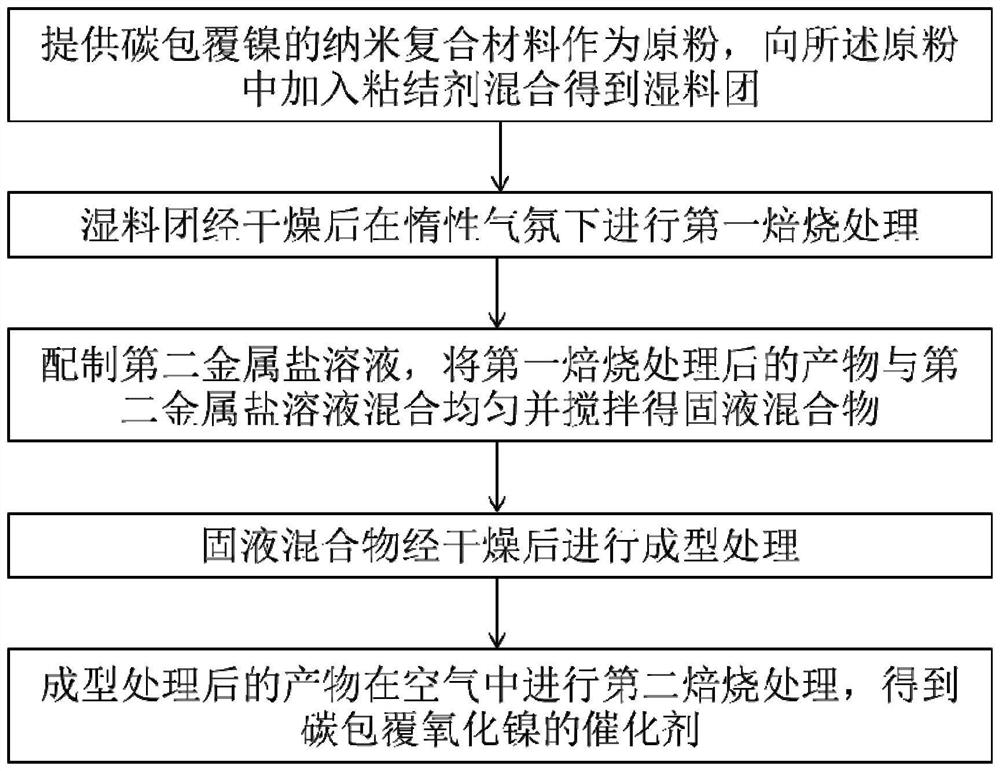
[0050] figure 1 It is a flow chart of the preparation process of a carbon-coated nickel oxide catalyst according to an embodiment of the present invention. Such as figure 1 As shown, the present invention provides a method for preparing a carbon-coated nickel oxide catalyst, comprising the following steps: providing a carbon-coated nickel nanocomposite as a raw powder, adding a binder to the raw powder and mixing to obtain a wet material Dough; the wet dough is dried and subjected to the first roasting treatment under an inert atmosphere; the second metal salt solution is prepared, and the product after the first roasting treatment is mixed with the second metal salt solution and stirred to obtain A solid-liquid mixture; the solid-liquid mixture is dried and subjected to molding treatment; and the product after the molding treatment is subjected to a second roasting treatment in air to obtain a carbon-coated nickel oxide catalyst; wherein the second metal salt solution selec...
Embodiment 1
[0095] This embodiment is used to illustrate the preparation method of catalyst of the present invention
[0096] (1) Weigh 10 g of nickel carbonate and 10 g of citric acid into a beaker containing 100 mL of deionized water, stir at 70° C. to obtain a homogeneous solution, and continue heating and evaporating to dryness to obtain a solid precursor.
[0097] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed in nitrogen gas with a flow rate of 100mL / min, and heat at a rate of 4°C / min. The temperature was raised to 600° C., and the heating was stopped after keeping the temperature for 2 hours, and cooled to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel nanocomposite material. According to elemental analysis, the mass percentages of elements contained in the carbon-coated nickel nanocomposite material are: carbon 26.14%, hydrogen 0.42%, oxy...
Embodiment 2
[0105] (1) Weigh 10g of nickel acetate and 10g of citric acid into a beaker containing 100mL of deionized water, stir at 70°C to obtain a homogeneous solution, continue heating and evaporate to dryness, and obtain a solid precursor.
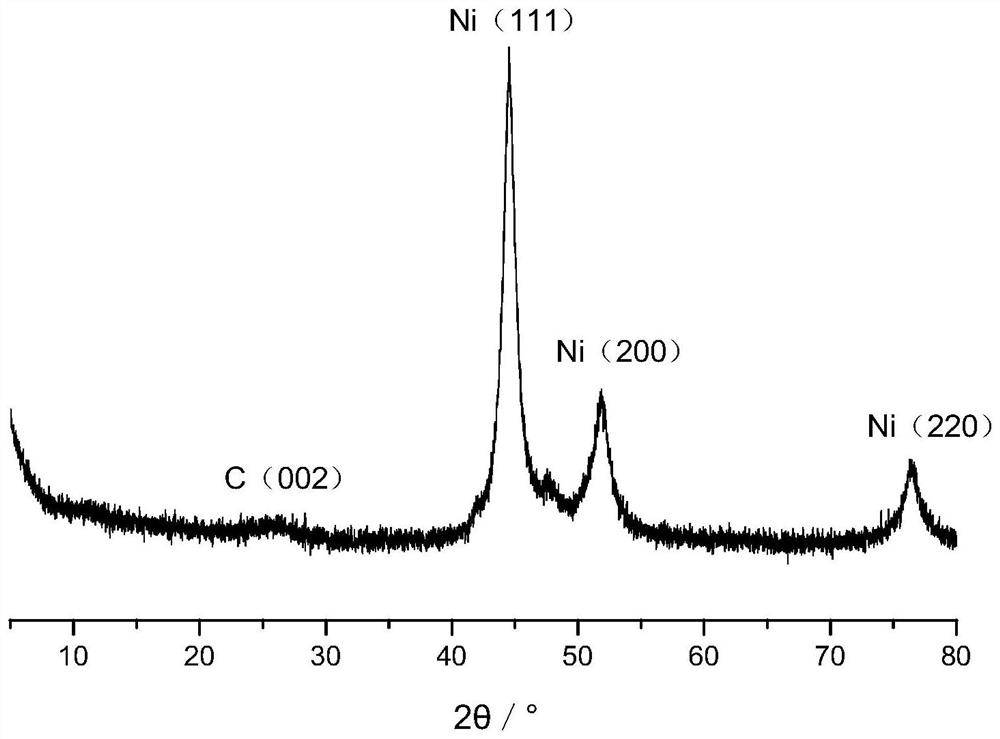
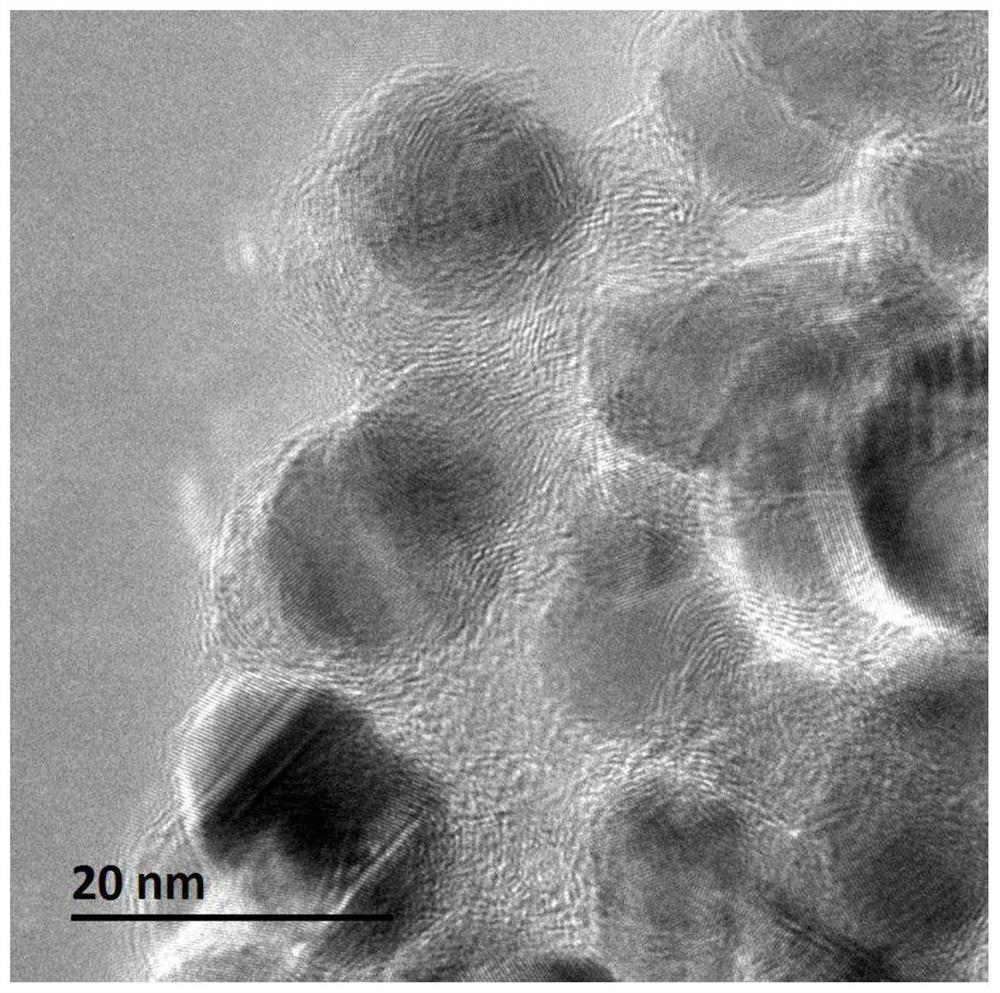
[0106] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed nitrogen gas with a flow rate of 100mL / min, and set the temperature at a rate of 2°C / min. Raise the temperature to 650° C., stop the heating after 2 hours of constant temperature, and cool to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel nanocomposite material. According to elemental analysis, the mass percentages of elements contained in the carbon-coated nickel nanocomposite are: carbon 24.29%, hydrogen 0.47%, oxygen 0.96%, nickel 74.28%. From Figure 4 It can be seen that the nickel in this material is present in a reduced state. Figure 5 It is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com