Method for degrading sulfamethoxazole by using biochar to activate bicarbonate peroxide
A technology of peroxybicarbonate and sulfamethoxazole, which is applied in the field of using biochar to activate peroxycarbonate to degrade sulfamethoxazole, can solve the problems of limited application and secondary pollution, and achieve high degradation efficiency, The effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of raw material biochar
[0035] The purchased wood chips, wheat stalks, corn stalks and bamboo were ground and passed through a 40-mesh sieve, washed repeatedly with deionized water, and dried at 80°C. Under oxygen-deficient conditions, heat up to 500°C in a muffle furnace at a heating rate of 10°C / min, and calcinate for 2 hours. After natural cooling, the obtained material was washed with ultrapure water until the water absorbance value of the soaked biochar at a wavelength of 245 nm was less than 0.05. The cleaned biochar was dried at 80°C, cooled and stored in an airtight manner.
Embodiment 2
[0036] Example 2 Biochar activates peroxybicarbonate
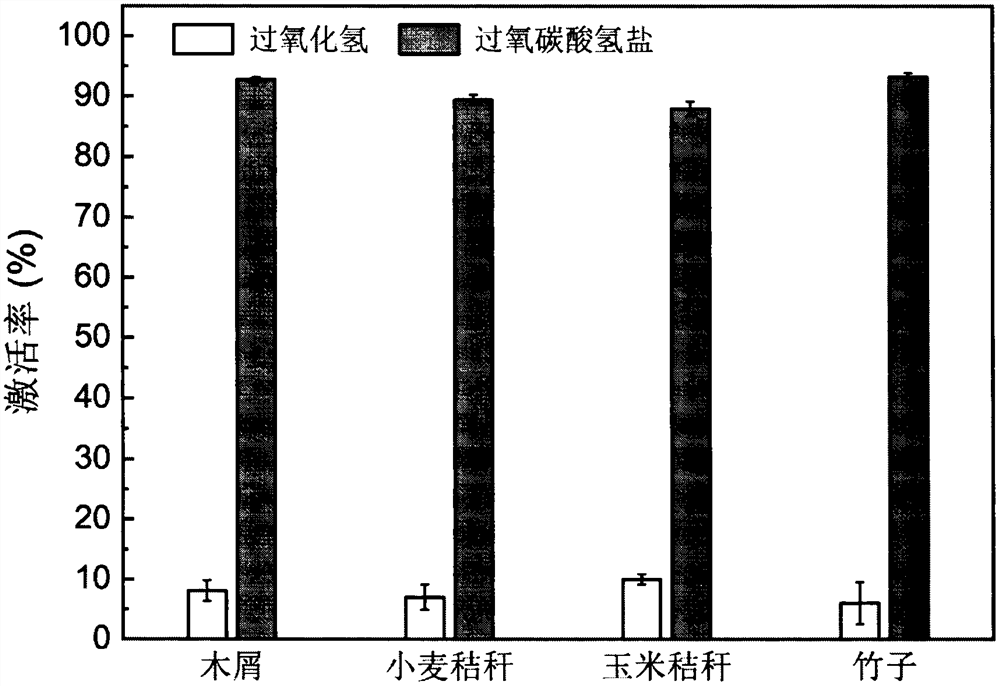
[0037] The biochar prepared in Example 1 was soaked in ultrapure water. After soaking for 24 hours, take 1g / L and add hydrogen peroxide to 10mM, sodium bicarbonate solution to 250mM, and disodium hydrogen phosphate to 10mM. Make the pH of the reaction system around 9. At room temperature (25±2° C.), the reaction was initiated and maintained as a homogeneous system by mixing the suspension at 200 rpm. At the beginning of the reaction and after 6 hours of reaction, take 1mL samples of the supernatant and quickly push them through a membrane filter holder equipped with a 0.45μm membrane to filter, add 1mL of 0.5M sulfuric acid, 0.1M titanyl sulfate, and 7.9mL of ultrapure water and shake well Immediately use a UV spectrophotometer to measure the absorbance at 410nm to obtain the absorbance of hydrogen peroxide. Since hydrogen peroxide and peroxybicarbonate are the relationship between the reactant and the product (see formu...
Embodiment 3
[0046] Example 3 Biochar activates peroxybicarbonate to remove sulfamethoxazole in water
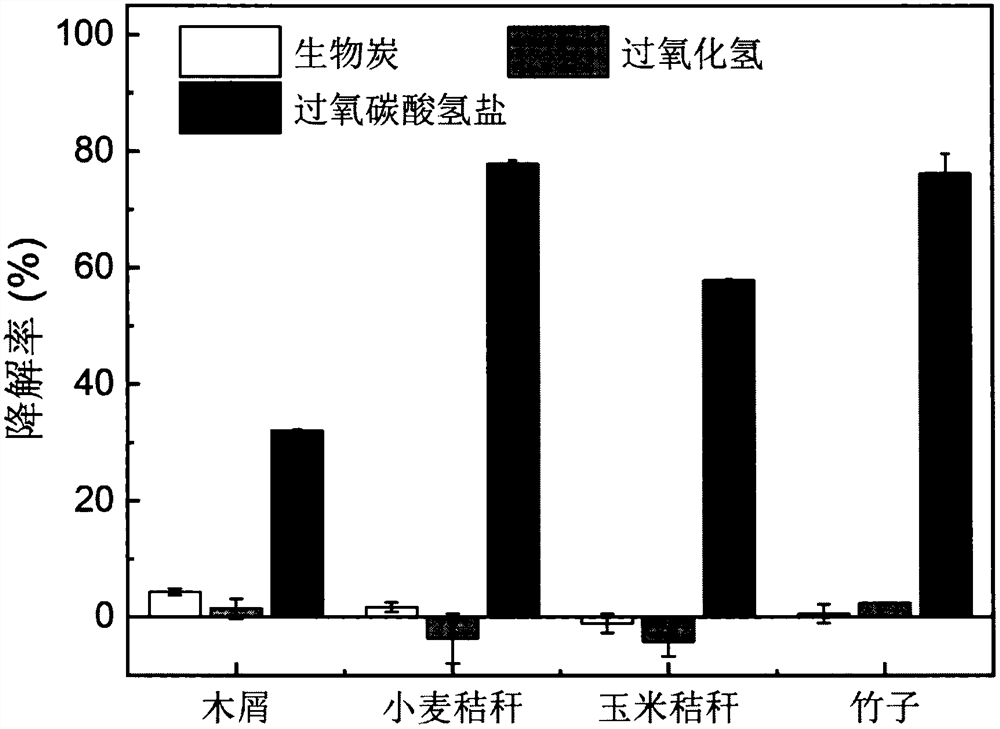
[0047] The biochar prepared in Example 1 was soaked in ultrapure water at 1 g / L. After soaking for 24 hours, add hydrogen peroxide to 10 mM, sodium bicarbonate solution to 250 mM, disodium hydrogen phosphate to 10 mM, and sulfamethoxazole to 20 μM. Make the pH of the reaction system around 9. At room temperature (25±2° C.), the reaction was initiated and maintained as a homogeneous system by mixing the suspension at 200 rpm. At the beginning of the reaction and after 6 hours of reaction, take 1mL samples of the supernatant and add the extract (0.1M NaOH:methanol=1:1), and shake the mixture at 200rpm for 10 minutes on a constant temperature shaker to make the SMX adsorbed on the biochar desorption. Then, the extracted mixture was quickly pushed through a membrane filter holder equipped with a 0.22 μm membrane into a high performance liquid chromatography (HPLC) vial using a 1 mL plasti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com