Method for synthesizing diflubenzuron by one-pot method
A technology of diflubenzuron and p-chloroaniline, applied in the field of pesticides, can solve the problems of difficult industrial production, harsh storage and transportation conditions, affecting yield and quality, etc., to save the separation and purification process, inhibit the generation of impurities, reduce The effect of the risk of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
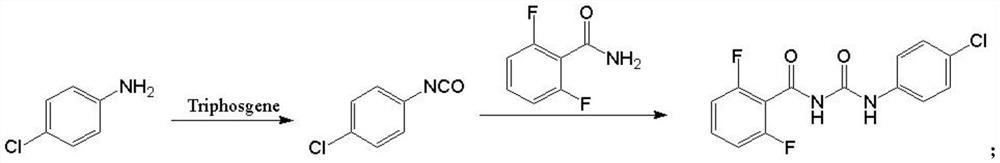
[0024] (1) Take 12.11g (0.041mol) of triphosgene in a 500ml there-necked bottle, add 36.33g of xylene, stir to dissolve, cool down to -5~5°C; take another 13.00g (0.102mol) of p-chloroaniline, add Dissolve 91g of xylene and add it dropwise to the triphosgene solution; control the temperature of the reaction system during the dropping process to -5-5°C;
[0025] (2) After the dropwise addition, add 0.39g (3.0wt%) of pyridine; then heat up to 25-35°C for 1h; continue to heat up to 80-90°C for 1h; take a sample to detect that the residue of p-chloroaniline is 0.2%, and the system is clear ;
[0026] (3) After the reaction, 16.51 g (0.105 mol) of 2,6-difluorobenzamide was added to the reaction solution, and then the temperature was raised to 142°C for reflux reaction; after 5 hours of heat preservation, HPLC detected that the intermediate p-chlorophenyl isocyanate remained 0.3 %, the reaction ends;
[0027] (4) Cool down and crystallize to obtain a crude product of diflubenzuron...
Embodiment 2
[0029] (1) Take 12.11g (0.041mol) of triphosgene in a 500ml there-necked bottle, add 36.33g of chlorobenzene, stir to dissolve, cool down to -5~5°C; take another 13.00g (0.102mol) of p-chloroaniline, add 104g of chlorobenzene was dissolved and added dropwise to the triphosgene solution; the temperature of the reaction system was controlled during the dropping process to be -5 to 5°C;
[0030] (2) After the dropwise addition, add 0.52g (4.0wt%) of triethylamine; then heat up to 25-35°C for 1h; continue to heat up to 80-90°C for 1h; sampling and detection of p-chloroaniline residue is 0.4%, system clarification;
[0031] (3) After the reaction, 16.19 g (0.103 mol) of 2,6-difluorobenzamide was added to the reaction solution, and then the temperature was raised to 130° C. for reflux reaction; after 6 hours of heat preservation, HPLC detected that the intermediate p-chlorophenyl isocyanate remained 0.8 %, the reaction ends;
[0032] (4) Cool down and crystallize to obtain a crude...
Embodiment 3
[0034] (1) Take 12.11g (0.041mol) of triphosgene in a 500ml three-necked bottle, add 48.44g of petroleum ether (boiling range 90-120°C), stir to dissolve, and cool down to -5-5°C; another 13.00g ( 0.102mol) of p-chloroaniline, add 143g of petroleum ether (boiling range 90-120°C) to dissolve, and add dropwise to the triphosgene solution; during the dropping process, the temperature of the reaction system is controlled at -5°C to 5°C;
[0035] (2) After the dropwise addition, add 0.65g (5.0wt%) of N'N-dimethylformamide; then heat up to 25-35°C for 1h; continue to heat up to 80-90°C for 1h; The residue of aniline is 0.2%, and the system is clear;
[0036] (3) After the reaction, 16.83 g (0.107 mol) of 2,6-difluorobenzamide was added to the reaction solution, and then the temperature was raised to 103° C. for reflux reaction; after 16 hours of heat preservation, HPLC detected that the intermediate p-chlorophenyl isocyanate remained 1.0 %, the reaction ends;
[0037] (4) Cool dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com