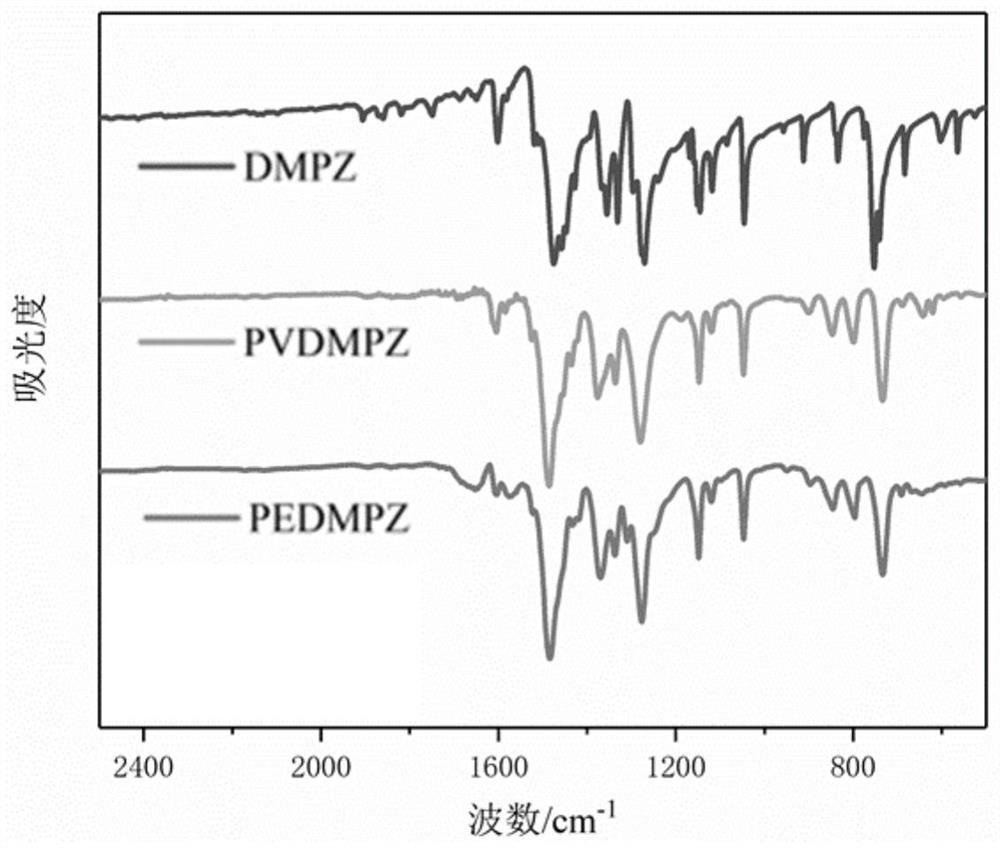
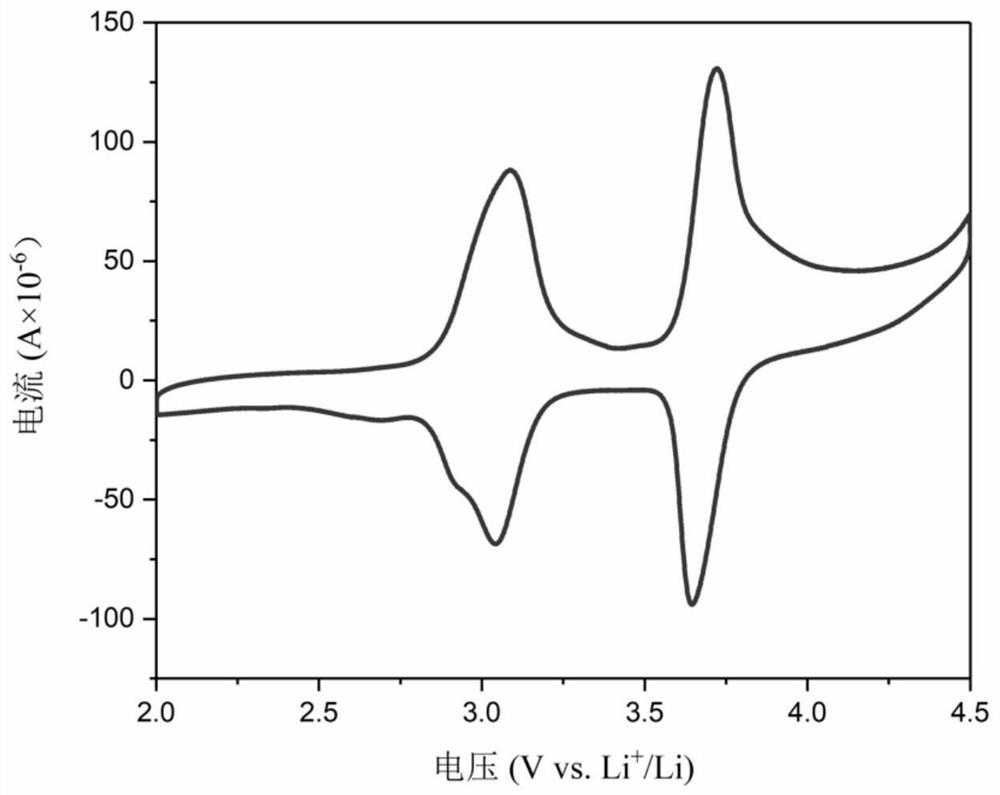
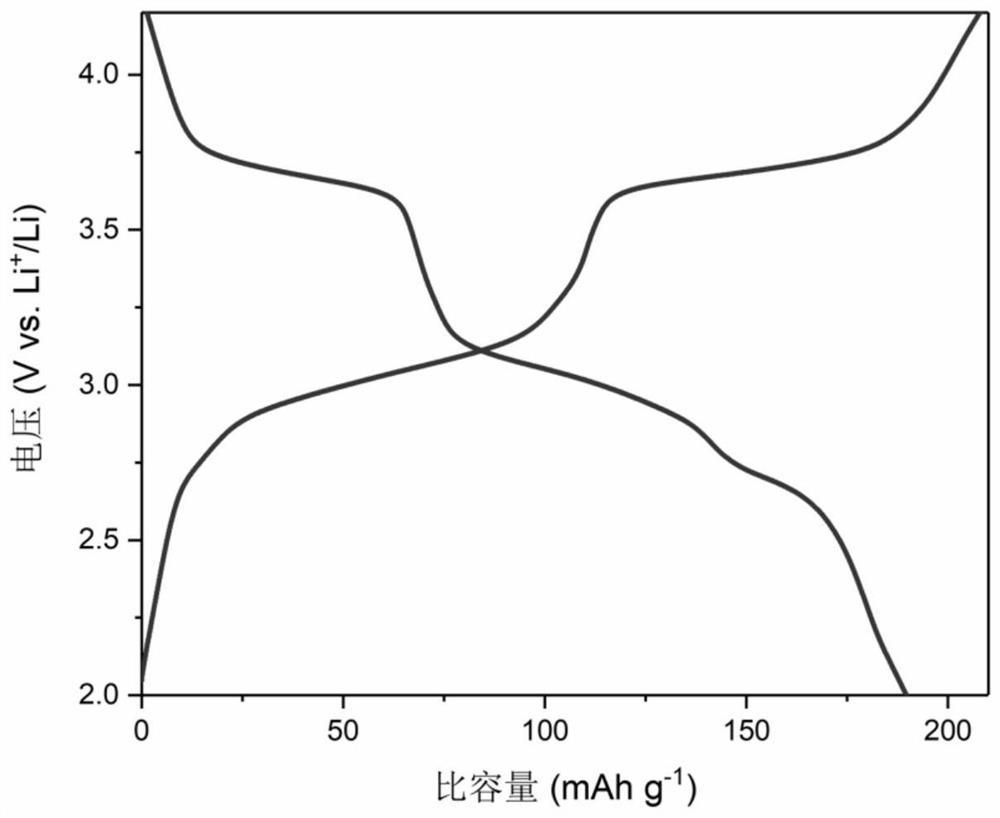
5, 10-dialkyl-5, 10-dihydrophenazine polymer as well as preparation method and application thereof
A technology of dihydrophenazine and dihydrophenazine, which is applied in the field of 5,10-dialkyl-5,10-dihydrophenazine polymers, can solve the problem of reducing the theoretical specific capacity of materials and poor battery cycle performance. It can achieve the effect of high cycle stability and high capacity ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of embodiment 1 poly(2-vinyl-5,10-dimethyl-5,10-dihydrophenazine)
[0061] Step 1: Synthesis of 2-bromo-5,10-dimethyl-5,10-dihydrophenazine (compound 2)
[0062]
[0063] Add 1 (0.89g, 3.45mmol) to a 250mL three-necked flask with a stirrer, set up a reflux device, pump nitrogen three times, then add 15mL ethanol with a syringe, heat and stir to dissolve. Sodium dithionite (7g, 34.5mmol) was dissolved in 130mL of distilled water, added to the system through a syringe, heated to about 120°C and refluxed for 4 hours to obtain a blue-green solid suspension. The solid was suction-filtered, washed with water, and freeze-dried to obtain 0.695 g of 2-bromo-5,10-dihydrophenazine intermediate. Add 2-bromo-5,10-dihydrophenazine (0.695g, 2.67mmol) into a 100mL three-necked flask with a stirring bar, pump nitrogen three times, then add 20mL ultra-dry tetrahydrofuran with a syringe, and set dry ice- The ethanol bath was cooled to -78°C. Add n-butyllithium (4.9mL, 5.4m...
Embodiment 2
[0075] Example 2 Synthesis of (2-ethynyl-5,10-dimethyl-5,10-dihydrophenazine)
[0076]Step 1: Synthesis of 2-ethynyl-5,10-dimethyl-5,10-dihydrophenazine (compound 4)
[0077]
[0078] Add compound 2 (28.9 mg, 1 mmol), cuprous iodide (3.8 mg, 20 μmol), bis(benzonitrile) palladium dichloride (21 mg, 30 μmol) into the Schlenk tube with a stirring bar, replace nitrogen three times, pass Add 0.12mL tri-tert-butylphosphine (10w / v%, 60μmol), 0.7mL trimethylsilylacetylene (490mg, 5mmol), 5mL dioxane and 3mL diisopropylamine into the syringe, and heat to 60°C for 48 hours . The resulting mixture was separated by silica gel column chromatography (eluent: n-hexane), and the intermediate product obtained was dissolved in 10 mL of tetrahydrofuran, and 1.2 mL of tetra-n-butylammonium fluoride (1mol / Lin THF, 1.2 mmol) was added, and stirred at room temperature 2 hours. The resulting solution was concentrated and separated by silica gel column chromatography (eluent: n-hexane) to obtain...
Embodiment 3
[0085] Embodiment 3 uses polymer P1 and P2 as the test of the electrochemical performance of lithium secondary battery cathode material
[0086] Preparation of the positive composite pole piece: when preparing the positive composite pole piece with P1 or P2 as the active material, 45mg of the active material P1 or P2 and 40mg of the conductive agent (Ketjen Black and / or Super P) were mixed together by manual grinding, Dissolve polyvinylidene fluoride (PVDF) in N-methylpyrrolidone (NMP), disperse the ground material in NMP, and stir at room temperature for 24 hours. Lay the current collector aluminum foil on a glass sheet, roll the above slurry into a film with a roller press, and dry it in vacuum at 70° C. for 12 hours. Use a microtome to press into an original sheet with a radius of 6 mm.
[0087] Assembly of button cell: take the composite film prepared above as the positive electrode, the lithium sheet as the negative electrode, and the Celgard2400 microporous membrane as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com