Oxidation catalyst as well as preparation method and application thereof
A technology of oxidation catalyst and catalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., which can solve the problem of powder or piece falling off and weak adhesion , affect the catalyst effect and long-term service life, etc., to achieve the effect of increasing the conversion rate and increasing the weight yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
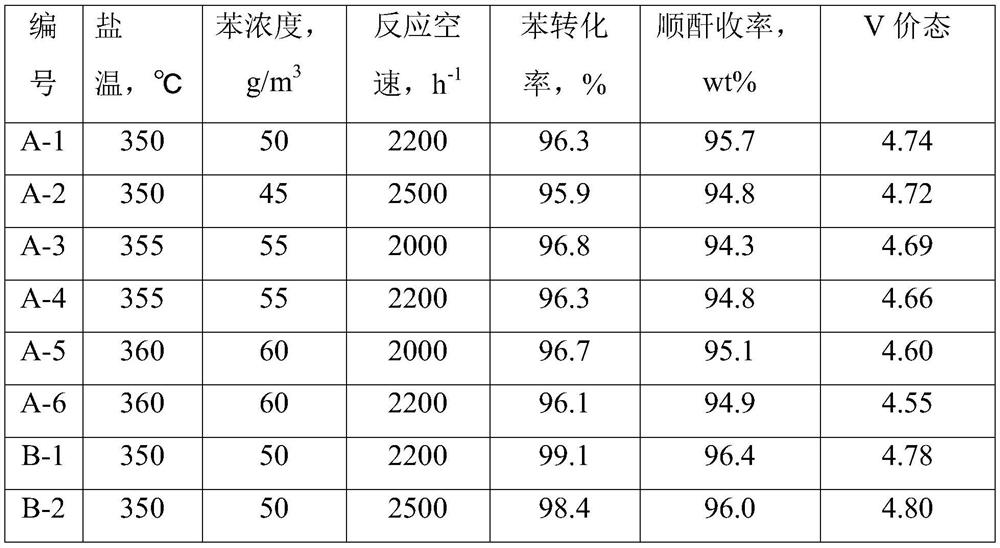
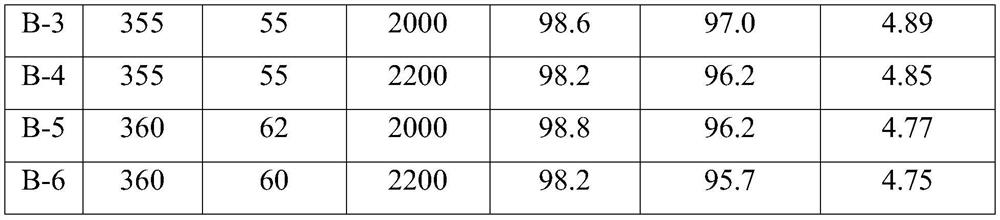
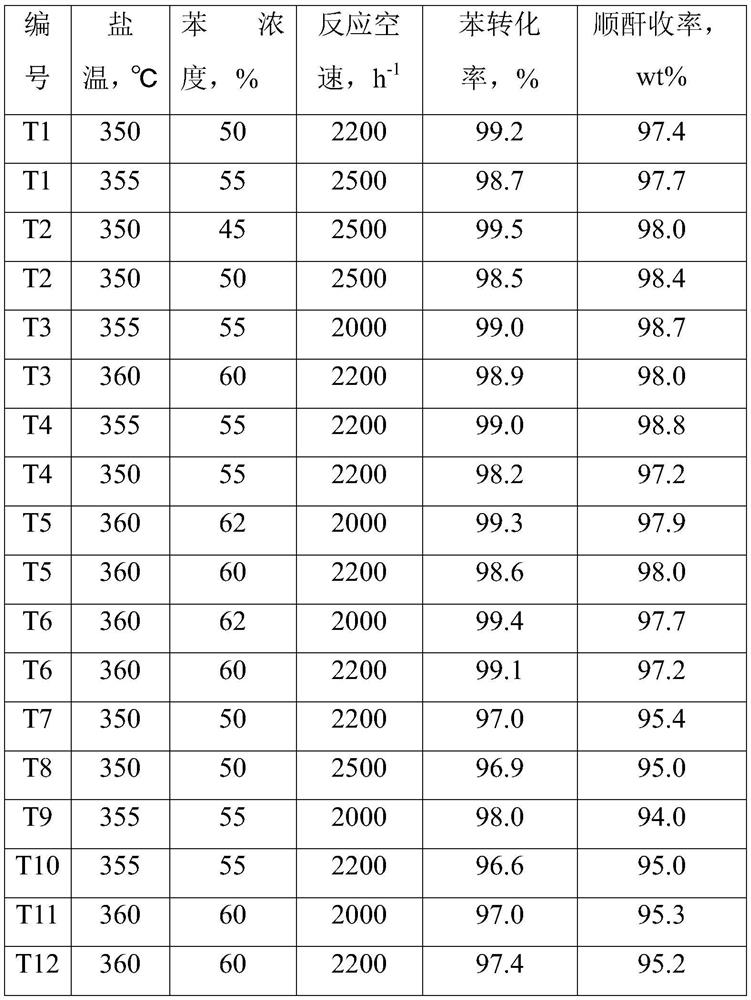
[0107] At room temperature, weigh 173.4g of oxalic acid (90.0g / mol) and dissolve it in 600mL of deionized water, and stir until dissolved; while stirring, add 112.7g of ammonium metavanadate (117.0g / mol) to it to obtain solution 1; Take by weighing 51.0g ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 , 1235.9g / mol) was dissolved in 100mL of deionized water at 50°C to obtain solution 2; 0.56g of nickel nitrate (290.7g / mol), 3.89g of iron nitrate (404.0g / mol), 0.29g of rubidium nitrate (147.7 g / mol), 2.37g trisodium phosphate (164.3g / mol) and 0.89g boric acid (61.8g / mol) were gradually added to 100 milliliters of deionized water at 50°C to obtain dispersion system 3; weighed 1.97g indium acetate ( 292.0g / mol) was added into 100mL of dilute acetic acid solution with a mass fraction of 5%, and stirred to obtain dispersion system 4; And 300g of sodium carboxymethyl cellulose solid content is 5% aqueous dispersion is poured into the colloid mill rapidly at the same time, mixed and emul...
Embodiment 2
[0111] At room temperature, weigh 177.7g of oxalic acid (90.0g / mol) and dissolve it in 600mL of deionized water, and stir until dissolved; while stirring, add 112.7g of ammonium metavanadate (117.0g / mol) to it to obtain solution 1; Take by weighing 34.0g ammonium molybdate ((NH 4 ) 6 Mo 7 o 24, 1235.9g / mol) was dissolved in 100mL of deionized water at 50°C to obtain solution 2; 1.79g boric acid (61.8g / mol), 1.40g nickel nitrate (290.7g / mol), 3.75g cesium nitrate (194.9g / mol), 1.40g cobalt nitrate (291.1g / mol) and 0.41g phosphorus pentoxide (141.9g / mol) were gradually added to 100 ml of deionized water at 50°C to obtain dispersion system 3; weigh 1.41g of indium acetate (292.0g / mol) was added into 100mL deionized water, stirred to obtain dispersion system 4; after solution 1 was reacted for 0.5 hours, solution 1, solution 2, dispersion system 3, dispersion system 4, 100mL formamide and 200g vinyl acetate - The mixed system of the aqueous dispersion with ethylene content of...
Embodiment 3
[0115] At room temperature, weigh 182.0g of oxalic acid (90.0g / mol) and dissolve it in 600mL of deionized water, and stir until dissolved; while stirring, add 112.7g of ammonium metavanadate (117.0g / mol) to it to obtain solution 1; Take by weighing 37.4g ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 , 1235.9g / mol) was dissolved in 100mL of deionized water at 50°C to obtain solution 2; 2.80g of nickel nitrate (290.7g / mol), 1.73g of magnesium nitrate (256.4g / mol), 0.78g of potassium nitrate (101.1 g / mol), 1.58g trisodium phosphate (164.3g / mol) and 1.96g cobalt nitrate (291.1g / mol) were gradually added to 100 ml of deionized water at 50°C to obtain dispersion system 3; weigh 0.78g pentoxide Diantimony (323.5g / mol) was added to 100mL of dilute nitric acid solution with a mass fraction of 10%, and stirred to obtain dispersion system 4; after solution 1 was reacted for 0.5 hours, solution 1, solution 2, dispersion system 3, dispersion system 4 , 100mL of pyrrolidone and 100g of an aqu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com