Chimeric enzyme, application thereof in-vitro one-step reaction synthesis of Cap0 mRNA and method
An RNA polymerase and co-reaction technology, applied in the field of molecular biology, can solve the problems of degradation loss of intermediate products, reduction of mRNA production efficiency, quality and yield, and cumbersome procedures, so as to save production process and cost, and simplify post-transcriptional modification , the effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides a preparation method of the chimeric enzyme, the preparation method comprising the following steps:
[0062] 1) co-transfecting the construct comprising the RNA polymerase domain and the construct comprising the capping enzyme catalytic domain into a host cell, and culturing the host cell;
[0063] 2) Extract the chimeric enzyme from the culture system.
[0064] The host cell is selected from mammalian cells, insect cells, bacteria or yeast. In one embodiment, the cells are E. coli cells. The cells can be wild type or artificially modified.
[0065] The Escherichia coli cells are BL21(DE3).
[0066] In one embodiment, after the construct comprising the RNA polymerase domain and the construct comprising the capping enzyme catalytic domain are co-transfected into host cells, an inducer and / or a low temperature is added to induce the host cell during the culture process. The cells express the chimeric enzyme.
[0067] The inducer is...
Embodiment 1
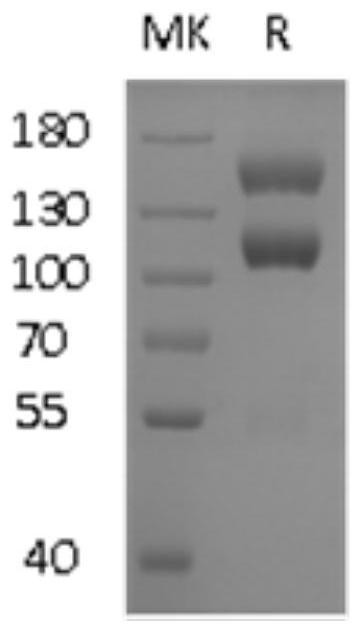
[0075] Embodiment 1: the preparation of the chimeric enzyme of T7 RNA polymerase and vaccinia RNA capping enzyme
[0076] 1. Vector construction and product expression of a chimeric enzyme of T7 RNA polymerase and vaccinia RNA capping enzyme
[0077] (1) Construction of T7 RNA polymerase gene fusion expression plasmid with Spy Catcher element and 6His
[0078] T7 RNA polymerase (T7RP) is fused to Spy Catcher gene by Linker1 to form recombinant plasmid A, the steps are as follows: first use primers pQE30-T7RP-F (SEQ ID NO: 1) and T7RP-R (SEQ ID NO: 2) to amplify T7 RNA polymerase fragments were extracted, and then the gene sequence of Linker1-Spy Catcher was amplified using T7RP-Linker1-F (SEQ ID NO: 3) and T7RP-SC-R (SEQ ID NO: 4). After the amplification is complete, use the PCR one-step directional cloning kit (produced by Suzhou Nearshore Protein Technology Co., Ltd.) splices the gene sequence of T7RP+Linker1+Spy Catcher and seamlessly clones the gene sequence into a lin...
Embodiment 2
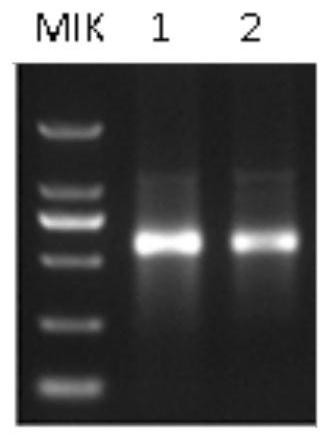
[0102] Example 2: Using a chimeric enzyme fused with T7 RNA polymerase and vaccinia RNA capping enzyme to obtain recombinant mRNA in one step
[0103] 1. Design of recombinant mRNA transcription template
[0104] In the present invention, the transcription template of the recombinant mRNA is a linearizable plasmid DNA, which is characterized by: containing T7 promoter, 5'UTR region, multiple cloning site region, 3'UTR region and polyadenylation Sequence and linearization site Esp3I. Polyadenylic acid sequence and linearization site: The polyadenylic acid sequence is composed of 150 consecutive adenosines. The sequence is synthesized by a third-party gene company, and a restriction endonuclease Esp3I site is added after the sequence Point: 5'-CGTCTC(N)1-3' / 3'-GCAGAG(N)5-5", the Esp3I sequence in the present invention is: 5'-AAAAAGAGACG-3' This site is used for the linearization of subsequent vectors And ensure that there is no nucleic acid residue at the PolyA tail after line...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com