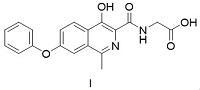
Crystallization process of Roxadustat bulk drug with controlled particle size
A technology for roxadustat and particle size control, which is applied in solution crystallization, crystallization condition screening, organic chemistry, etc., can solve the problems of fast dissolution, large particle size, and fine particle size, and achieve safe and simple crystallization process, high yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
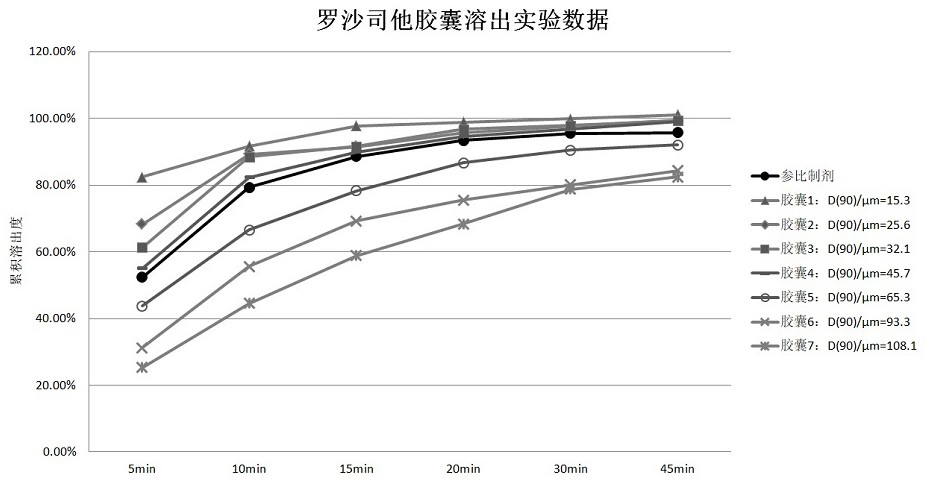
[0032] Embodiment 1. Dissolution contrast experiment of prescription preparations of different particle size raw materials
[0033] The effect of particle size of roxadustat raw material on the dissolution rate of capsules and the cumulative dissolution rate at the end point of dissolution was investigated.
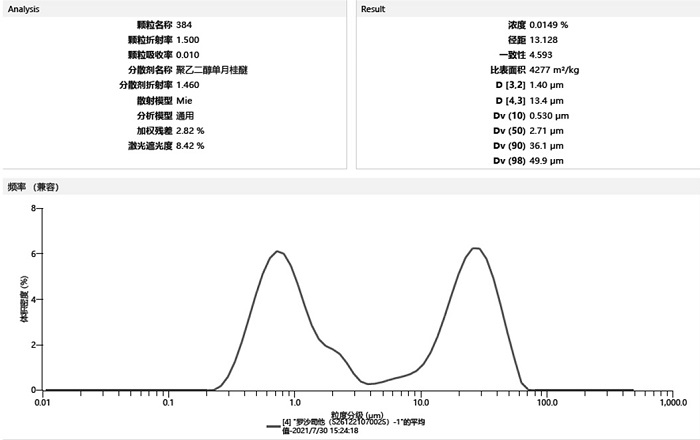
[0034] 1) By adjusting the crystallization process and combining pulverization means to obtain roxadustat APIs with different particle sizes, as shown in Table 1:
[0035] Table 1
[0036] .
[0037] 2) Make roxadustat raw materials with different particle sizes into capsules according to the following process:
[0038] Mix roxadustat with lactose, microcrystalline cellulose, croscarmellose sodium, and povidone through a cone granulator, stir and mix in a wet granulation pot, and then stir at 200 rpm, Under the condition of cutting at 1500 rpm, add purified water to granulate, dry in a fluidized bed, granulate with a 0.8 mm sieve, add magnesium stearate for total mix...
Embodiment 2
[0050] Embodiment 2, crystallization condition screening
[0051] (a) Temperature screening
[0052] During the crystallization process, under the same stirring speed, the effect of different dropping temperature on the particle size was investigated.
[0053] Put roxadustat (I) and water in a reaction kettle, add aqueous sodium hydroxide solution, dissolve roxadustat (I), filter, place the mother liquor in a crystallization kettle, raise the temperature to different temperatures, and drop into the first Part of the acetic acid aqueous solution, adjust the pH to 6.6; add roxadustat seed crystals, and quickly add the second part of the acetic acid aqueous solution, and keep stirring the system; centrifuge while hot, dry the filter cake to obtain the raw material drug of roxadustat, and measure the particle size after sampling , the experimental results are shown in Table 3:
[0054] table 3
[0055] .
[0056] Conclusion: During the crystallization process, under the same...
Embodiment 3
[0073]
[0074] Put 5.3 Kg of 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid methyl ester (compound II-1), 5.83 Kg of sodium glycinate and 51 Kg of methanol in the reactor for reflux reaction for 12 hours , cooling and filtering, the filter cake was dissolved in ethyl acetate and water and then separated, the water layer was warmed up to 70°C, and acetic acid aqueous solution was added, and after cooling down, it was centrifuged to obtain the wet crude product of roxadustat. Add the wet crude product of roxadustat into water, add sodium hydroxide aqueous solution, and filter the system. After the filtrate is warmed up to 65-75 °C, add acetic acid aqueous solution, filter while hot and dry to obtain 55.6 Kg of roxadustat (I), with a purity of: 99.5%, yield: 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com