Preparation method of 4-bromo-2, 5-dichlorobenzoic acid
A technology of dichlorobenzoic acid and dichlorotoluene is applied in the preparation of carboxylates, the preparation of organic compounds, and the preparation of carboxylic acids by oxidation, which can solve problems such as non-marketable supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
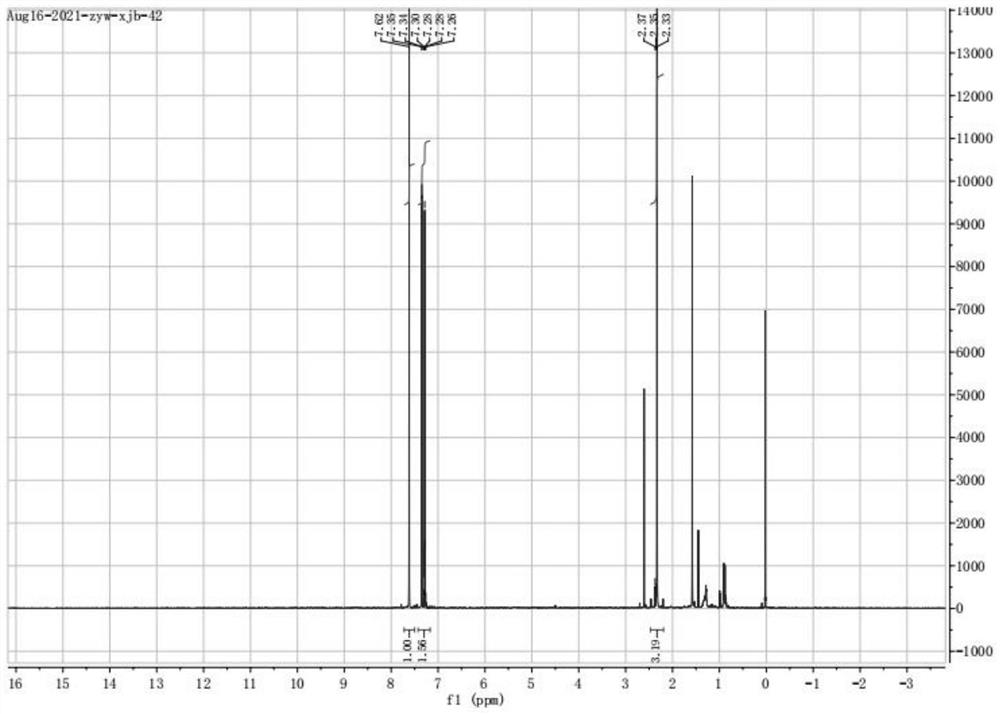
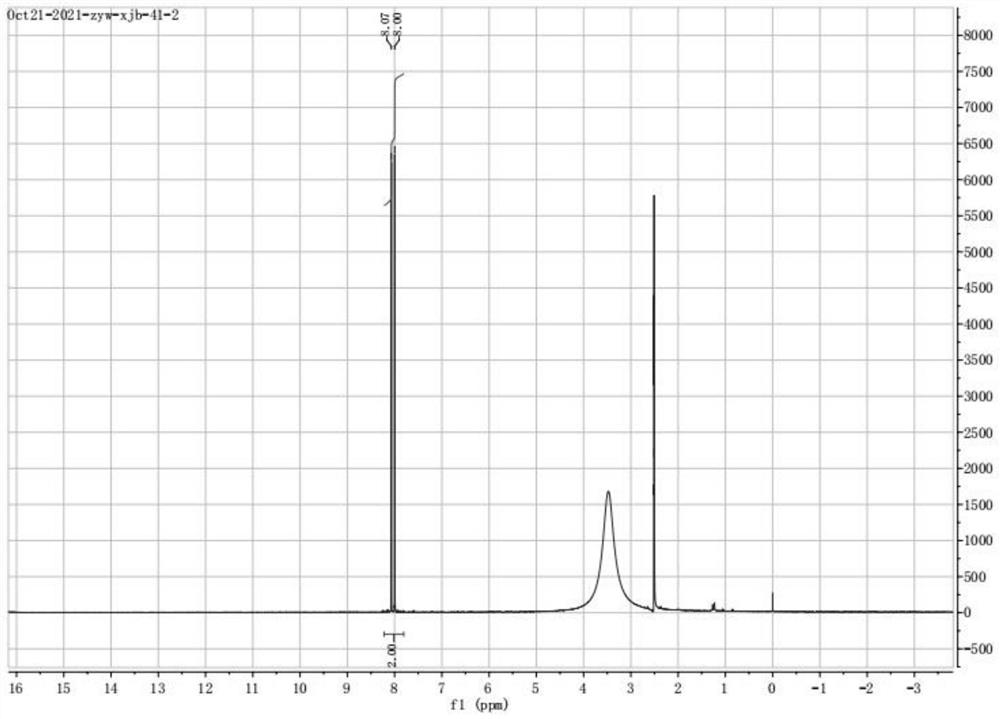
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of 4-bromo-2,5-dichlorobenzoic acid, comprising the following steps:
[0028] (1) Mix 2,5-dichlorotoluene, a brominating reagent, a catalyst, and a first solvent to perform a bromination reaction to obtain 4-bromo-2,5-dichlorotoluene;
[0029] (2) Mix the 4-bromo-2,5-dichlorotoluene, the oxidizing agent and the second solvent, and carry out an oxidation reaction to obtain 4-bromo-2,5-dichlorobenzoic acid.
[0030] The invention mixes 2,5-dichlorotoluene, a bromination reagent, a catalyst and a first solvent to carry out a bromination reaction to obtain 4-bromo-2,5-dichlorotoluene. In the present invention, the brominated reagent is preferably hydrobromic acid, hypobromous acid, N-bromosuccinimide, bromooctane, liquid bromine, N-bromoacetamide, trimethylbromosilane, N -One or more of bromosuccinimide, 1,3-dibromo-5,5-dimethylhydantoin and benzyl ammonium tribromide. In the present invention, the molar ratio of the 2,5...
Embodiment 1
[0056] React according to the equation shown in formula I:
[0057]
[0058] Before the reaction, cool the reaction kettle to 5°C, add 30g of 2,5-dichlorotoluene solution, 200mL of dichloromethane and 2g of ferric chloride into the reaction kettle, start stirring, and in the stirring state at 0.1mL / min Add 10mL of N-bromosuccinimide dropwise to the solution at a rate of 1.5h, and the dropwise addition is completed in about 1.5h, during which the temperature is controlled not to exceed 7°C. After the addition was completed, the reaction was stirred at 5°C for 1 h. After the reaction was completed, the reaction solution was washed three times with water, and then washed three times with saturated sodium bicarbonate solution until the solution became colorless. The solution was separated with a separatory funnel to obtain 4- Organic solution of bromo-2,5-dichlorotoluene. Anhydrous sodium sulfate was added to the solution for drying, and the organic solvent was removed by rota...
Embodiment 2
[0063] React according to the equation shown in formula II:
[0064]
[0065] Before the reaction, cool the reactor to 3°C, add 16g of 2,5-dichlorotoluene solution, 100mL of acetonitrile and 1g of iron into the reactor, start stirring, and pour the solution into the solution at a rate of 0.3mL / min under stirring. 5.5mL hypobromous acid was added dropwise to the solution, and the dropwise addition was completed in about 1 hour, during which the temperature was controlled not to exceed 5°C. After the addition was completed, the reaction was stirred at 3°C for 1.5 h. After the reaction was completed, the reaction solution was washed three times with water, and then washed three times with saturated sodium bicarbonate solution until the solution became colorless. The solution was separated with a separatory funnel to obtain 4 - Organic solution of bromo-2,5-dichlorotoluene. Solid calcium oxide was added to the solution for drying, and the organic solvent was removed by rotar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com