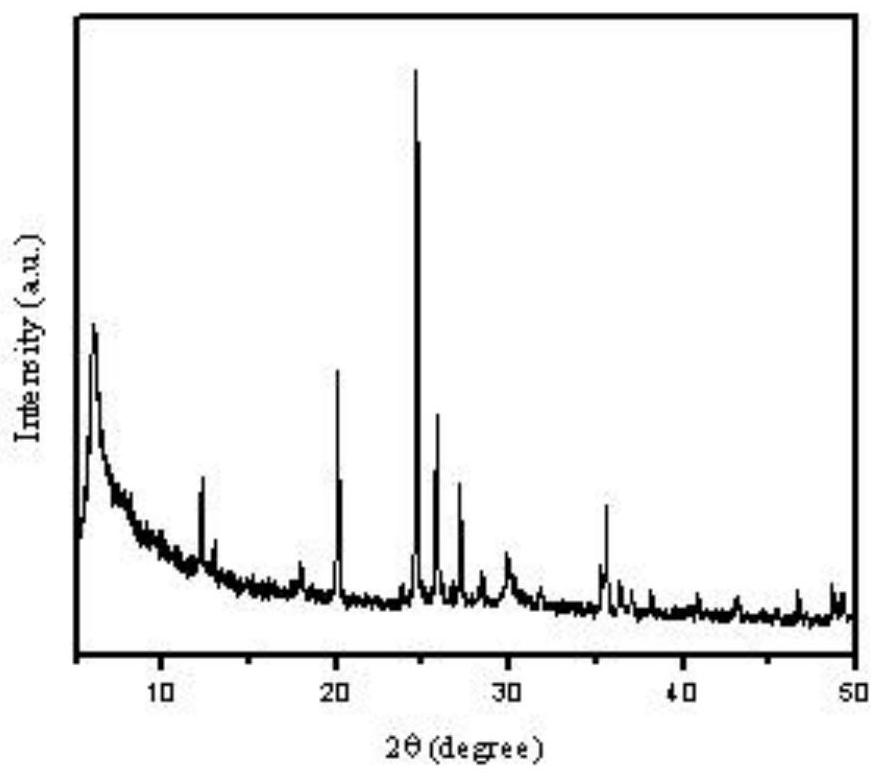
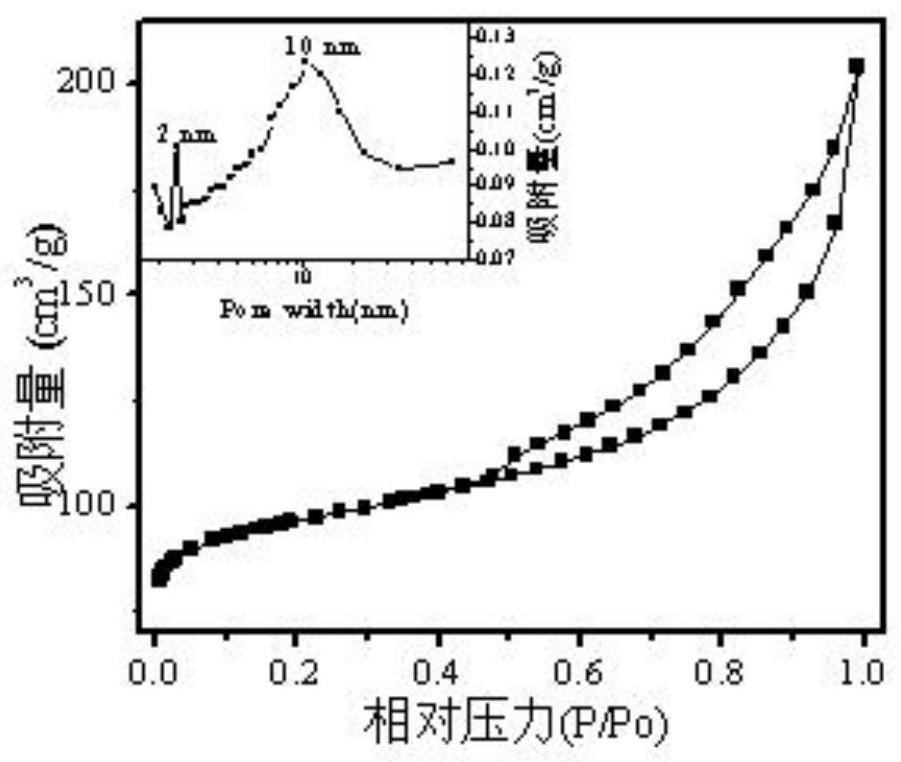
Preparation method and application of porous zeolite adsorbent
A technology of porous zeolite and adsorbent, which is applied in the field of preparation of porous zeolite adsorbent, can solve the problem of inability to enter zeolite, etc., and achieves the effects of high crystallinity, enhanced adsorption treatment, and large external surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The invention provides a preparation method of a porous zeolite adsorbent, the specific preparation steps are as follows:
[0030] Dissolve 5.0g of sodium hydroxide in 10g of deionized water, after fully dissolving, add 4.3g of solid silica gel, stir for 30 minutes, and record it as solution A; dissolve 1.6g of potassium fluoride in 24.1g of deionized water, fully dissolve, record Solution B; Mix solutions A and B, mix well, slowly add 12g of titanium trichloride solution and continue to stir for 2h; add 3.2mL of template agent and 9.86g of supplement to the above solution, stir for 3 hours, and obtain the reaction mixture. Glue, put in a kettle, and statically crystallize at 230°C for 72 hours; the crystallization is completed, and the sample is obtained after washing, suction filtration, drying, and calcination at 450°C for 5 hours; the molar ratio of each material of the obtained reaction mixture gel is 5.4 silica: Titanium dioxide: 4.5 Sodium oxide: 2.0 Potassium fl...
Embodiment 2
[0032] Compared to Example 1, no supplements were used in the preparation method.
Embodiment 3
[0034]Dissolve 5.2g of sodium hydroxide in 10g of deionized water, after fully dissolving, add 4.6g of solid silica gel, stir for 30 minutes, and record it as solution A; dissolve 1.9g of potassium fluoride in 26.5g of deionized water, fully dissolve, record Solution B; Mix solutions A and B, mix well, slowly add 12g of titanium trichloride solution and continue to stir for 2h; add 3.7mL of template agent and 9.86g of supplement to the above solution, stir for 3 hours, and obtain the reaction mixture. Glue, put in a kettle, and statically crystallize at 200°C for 82 hours; the crystallization is completed, and the sample is obtained after washing, suction filtration, drying, and calcination at 450°C for 5 hours; the molar ratio of each material of the obtained reaction mixture gel is 5.8 silica: Titanium dioxide: 4.7 Sodium oxide: 2.5 Potassium fluoride: 180 Deionized water: 1.5 Methacryloyloxyethyltrimethylammonium chloride: 0.7 Supplement; graphene, fluorine-containing hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com