Degradable silicon-based carrier material based on ZnO composite mesoporous silica as well as preparation method and application of degradable silicon-based carrier material
A technology of mesoporous silica and composite silica, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Enhanced aggregation, enhanced targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] In this example, a degradable silicon-based carrier material based on ZnO composite mesoporous silica was prepared.
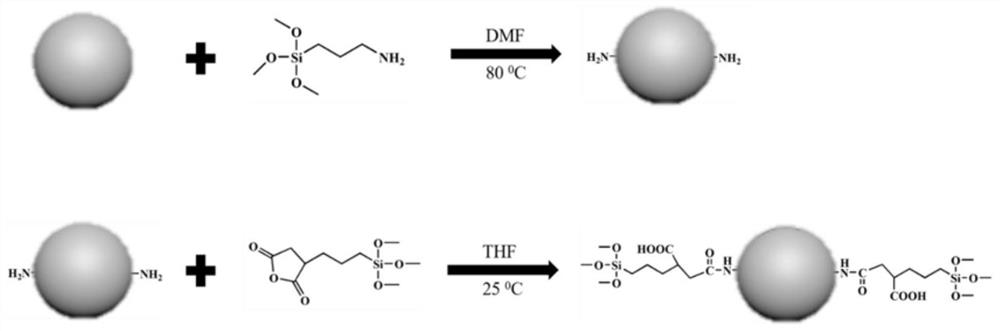
[0067] The synthesis route of ZnO composite mesoporous silica nanoparticles (ZnO@MSN) is as follows: figure 1 As shown, the ZnO quantum dots were firstly modified on the surface, and then reacted with 3-aminopropyltriethoxysilane (APTES) to obtain aminated ZnO quantum dots (ZnO-NH 2 ), followed by the reaction of aminated ZnO quantum dots with [3-(methoxysilane) propyl] succinic anhydride to obtain ZnO quantum dot silane coupling agent (ZnOTMS). Using cetyltrimethylammonium bromide (CTAB) as a template, adding a homogeneous mixture of tetraethyl orthosilicate (TEOS) and ZnOTMS, hydrolyzed to obtain ZnO@MSN.
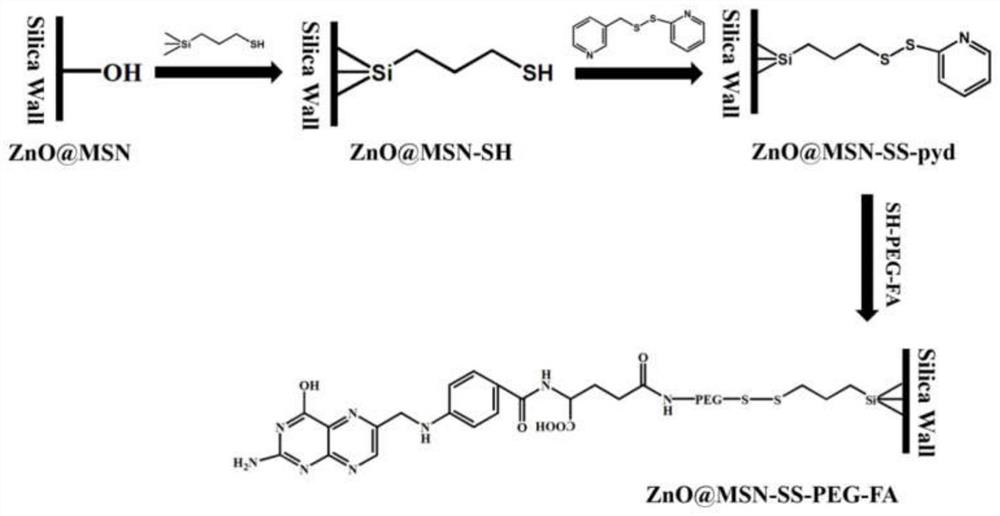
[0068] The synthetic route of degradable silicon-based carrier material (ZnO@MSN-SS-PEG-FA) based on ZnO composite mesoporous silica is as follows: figure 2 As shown, ZnO@MSN reacted with 3-mercaptopropyltrimethoxysilane (MPTS) to obtain mercapto-m...
Embodiment 2
[0090] In this example, the anticancer drug doxorubicin (DOX) was loaded on the ZnO@MSN-SS-PEG-FA prepared in Example 1, and the steps were as follows:
[0091] Add 10mg of ZnO@MSN-SS-PEG-FA into the DOX solution with a concentration of 2mg / mL, shake and stir at 50°C for 4h under dark conditions, so that DOX molecules enter the pores of ZnO@MSN-SS-pyr , solid-liquid separation, collecting particles, washing with pure water several times to remove DOX on the surface of ZnO@MSN-SS-pyr, and dispersing the obtained drug-loaded ZnO@MSN-SS-pyr in a PBS buffer solution with a pH value of 8.07 , add SH-PEG3400-FA, the mass ratio of ZnO@MSN-SS-pyr to SH-PEG3400-FA is 1:1, react at room temperature for 12h, separate solid and liquid, and wash with water to obtain drug-loaded nanoparticles DOX@ZnO@MSN -SS-PEG-FA.
[0092] Calculate the amount of free drug in the solution (recorded as free amount) by measuring the absorbance of the eluate at 481nm, and calculate the drug loading of DOX@Z...
Embodiment 3
[0096] In this example, performance tests were performed on the products prepared in each step in Example 1.
[0097] 1. Morphological analysis
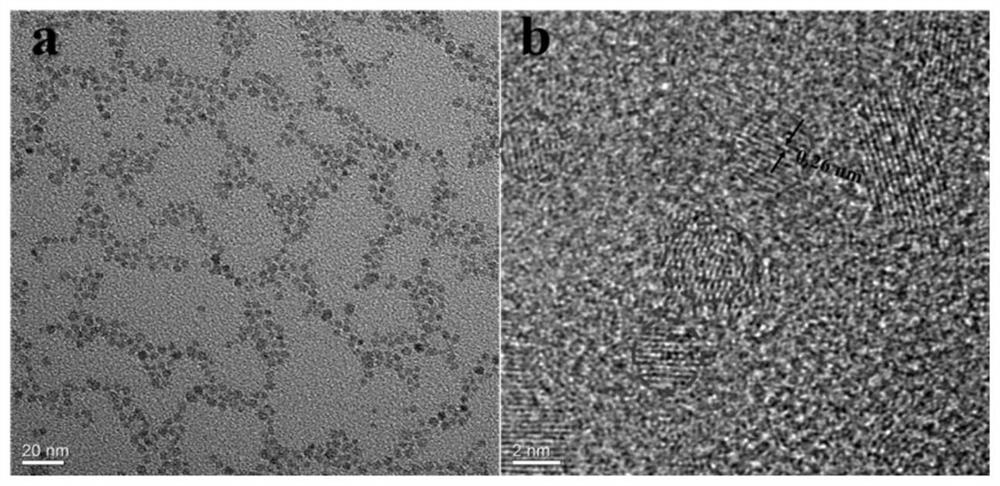
[0098] Observation step ZnO-NH by transmission electron microscope (TEM) 2 microstructure, the results are as follows image 3 Shown, ZnO-NH 2 The average particle size of ZnO quantum dots is 3.6±0.3nm, and obvious lattice fringes are observed, and the fringe spacing is 0.26nm, which is consistent with the (001) crystal plane of ZnO quantum dots.
[0099] Observing the microstructure of ZnO@MSN by TEM, the results are as follows Figure 4 As shown, ZnO@MSN has a spherical or ellipsoidal structure with uniform size and good dispersion. The average particle size of ZnO@MSN is 102±2nm, and its pores are complete and show through-shaped pores.
[0100] In order to explore the distribution of elements in ZnO@MSN, Si, O, C, N and Zn elements were analyzed by scanning transmission microscopy and energy dispersive X-ray spectroscopy (STE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com