Nickel-based self-supporting water electrolysis catalyst and preparation method thereof
A catalyst, self-supporting technology, applied in the field of electrocatalysis, can solve the problems of limited electrocatalytic performance, poor conductivity of hydroxide, large polarization, etc., to achieve controllable product morphology, lower energy barrier, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A nickel-based self-supporting electrolytic water catalyst, the preparation process of which comprises the following steps:
[0028] (1) 0.582g Ni(NO 3 ) 2 ·6H 2 O, 0.26g Ce(NO 3 ) 3 ·6H 2 O, 0.37g ammonium fluoride, 0.6g urea (all chemical reagents were purchased from Aladdin Ltd (China)) were dissolved in 24mL deionized water to obtain a mixed solution, which was then transferred to a 50mL autoclave liner after magnetic stirring for 30min. Then put a 2cm×2cm clean carbon cloth (Carbon Cloth, CC, thickness 0.33mm, purchased from CeTech Co., Ltd., model WOS1009) after being treated with acetone, ethanol and deionized water and dried into the above solution middle. Seal the liner and put it into a stainless steel autoclave, set up an electric heating constant temperature blast drying oven (DHG-9036A) and react at 120°C for 6 hours to obtain Ni(OH) 2 / CeO 2 / CC composites were rinsed repeatedly with deionized water and dried in a vacuum oven (DZF-6020) for later u...
experiment example 1
[0039] Characterization of Physicochemical Properties of Nickel-Based Self-supporting Catalysts for Electrolysis of Water
[0040] The nickel-based self-supporting electrolytic water catalyst obtained in Example 1 of the present invention is systematically studied on its composition, chemical bond, morphology and microstructure by modern nano-testing and analysis techniques such as XRD, XPS, SEM, and TEM, and the results are as follows:
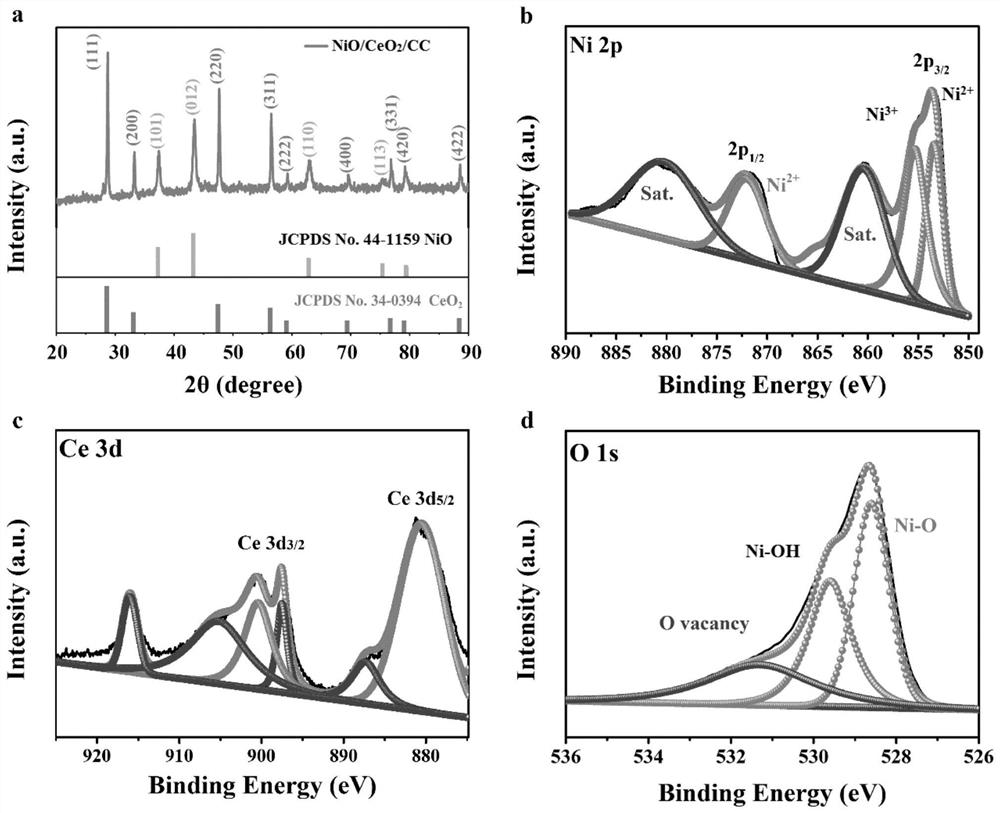
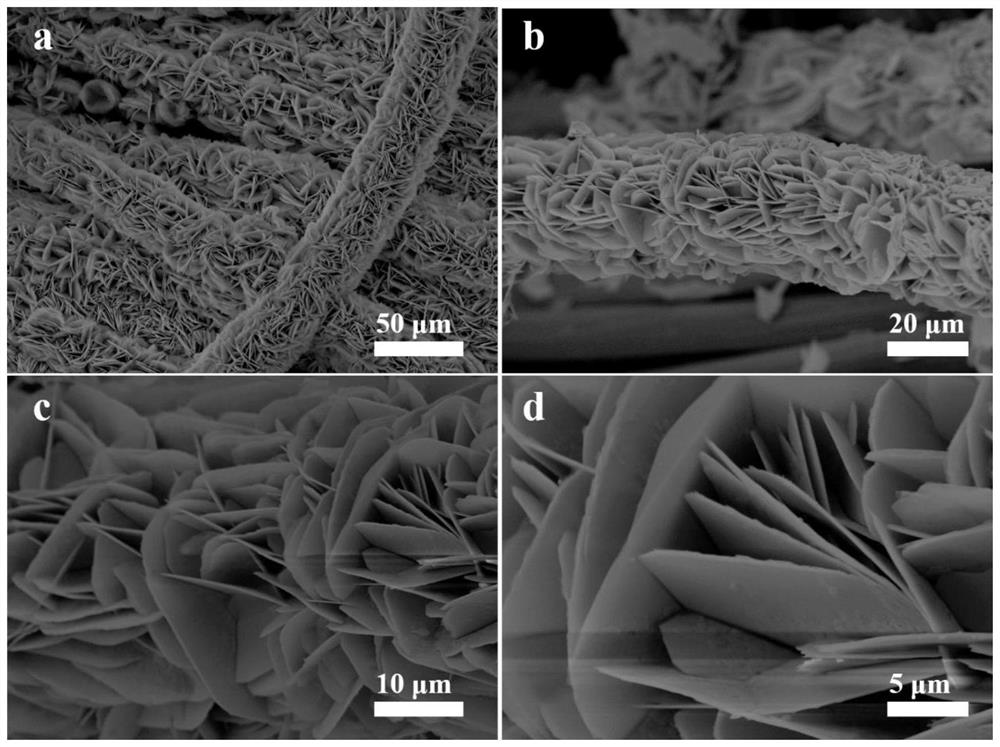
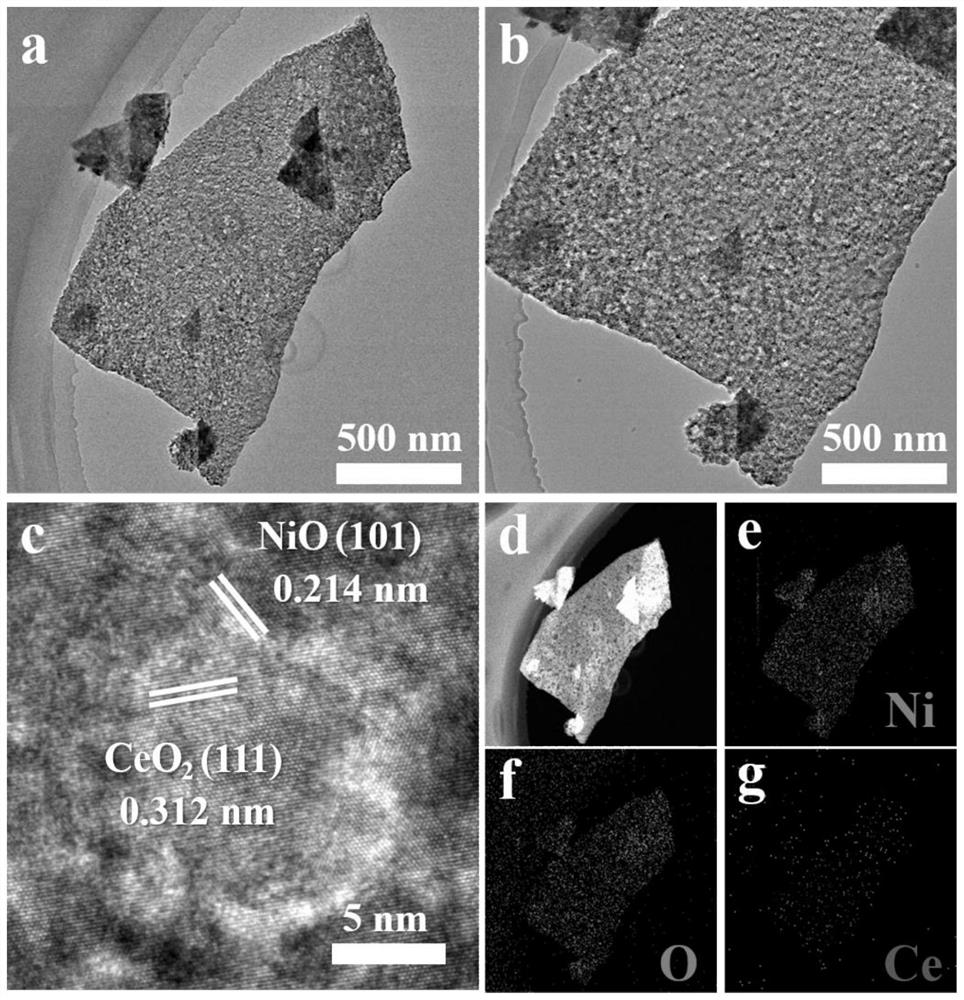
[0041] First the product that embodiment 1 obtains carries out XRD characterization ( figure 1 a), and compare the XRD pattern with NiO, CeO 2 Standard card correspondence. The result shows that the product that embodiment 1 obtains contains NiO, CeO 2 Characteristic peaks, proving that the final product is NiO and CeO supported on carbon cloth 2 composite material. For determining the chemical bonding state of each element in the product, the product that embodiment 1 obtains has carried out XPS analysis ( figure 1 b-1d), XPS spectrogra...
experiment example 2
[0044] The products obtained in Example 1 and Comparative Examples 1 to 2 were used as catalysts to carry out electrolysis water hydrogen evolution and oxygen evolution tests respectively in a solution with electrolyte 1.0M KOH. The electrochemical measurement is carried out on an electrochemical workstation (CHI660E), using a standard three-electrode system, wherein the Hg / HgO electrode is the reference electrode, and the graphite electrode is the counter electrode. The products prepared in Example 1 of the present invention and Comparative Examples 1 to 2 are Working electrode (geometric area 1cm×1cm). Linear Sweep Voltammetry (LSV) at N 2 5mVs in saturated electrolyte -1 The scan rate is measured, Equation E vs.RHE =E vs.Hg / HgO +0.059*pH+0.098 Calculated potential vs. potential of the reversible hydrogen electrode (RHE). At a current density of 10mA cm -2 Under the condition of , the electrochemical stability was tested by chronopotentiometry curve.
[0045] The resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com