Nucleic acid drug delivery system, preparation method, pharmaceutical composition and application
A nucleic acid drug and delivery system technology, used in drug combinations, nano-drugs, pharmaceutical formulations, etc., can solve the problems of limiting the practical application of nucleic acid drugs, high cytotoxicity, and immune system clearance, and achieve safe and efficient intracellular drug delivery. Encapsulation efficiency, stability and safety enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of Epigallocatechin Gallate-Fe 3+ / siRNA core
[0047] Epigallocatechin gallate (EGCG) and FeCl 3 ·6H 2 O was prepared as a stock solution with RNase Free water, and siRNA was prepared as a 20 μM solution with RNase Free water. Vortex siRNA and EGCG solution for 2 hours, then mix briefly under vortex or quickly mix Fe by microfluidics 3+ solution, Fe 3+ The molar ratio to EGCG is 2:1. Incubate at room temperature for 30 minutes.
[0048] Specifically, Zetasizer 3000HS instrument particle size analyzer (Malvern Instruments, Malvern, UK) was used to measure the sample particle size and polydispersity index (PDI). At the same time, the loading efficiency of siRNA was examined by 1% agarose gel electrophoresis.
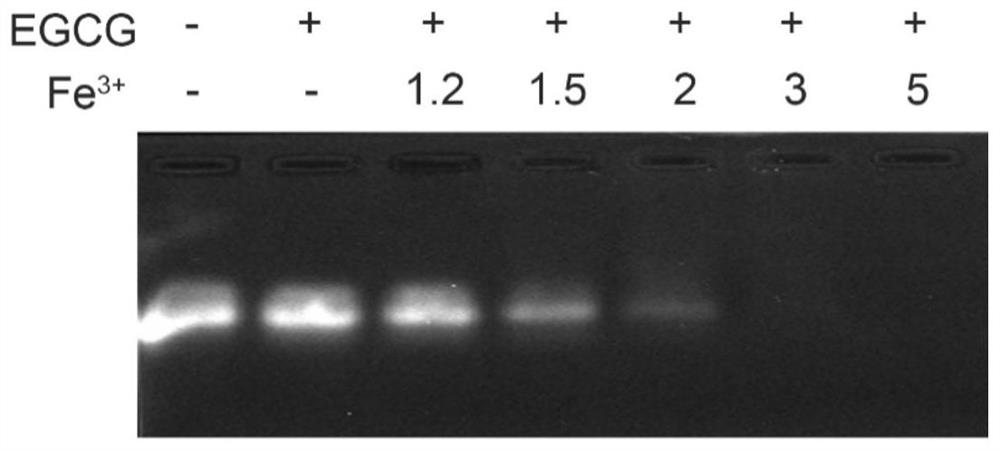
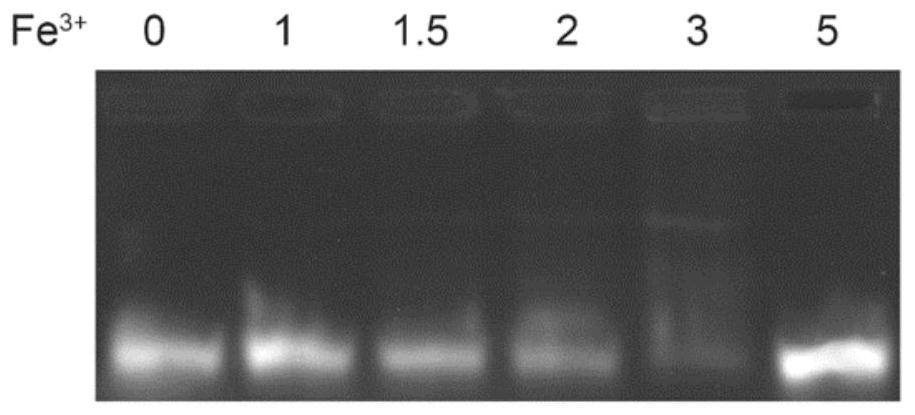
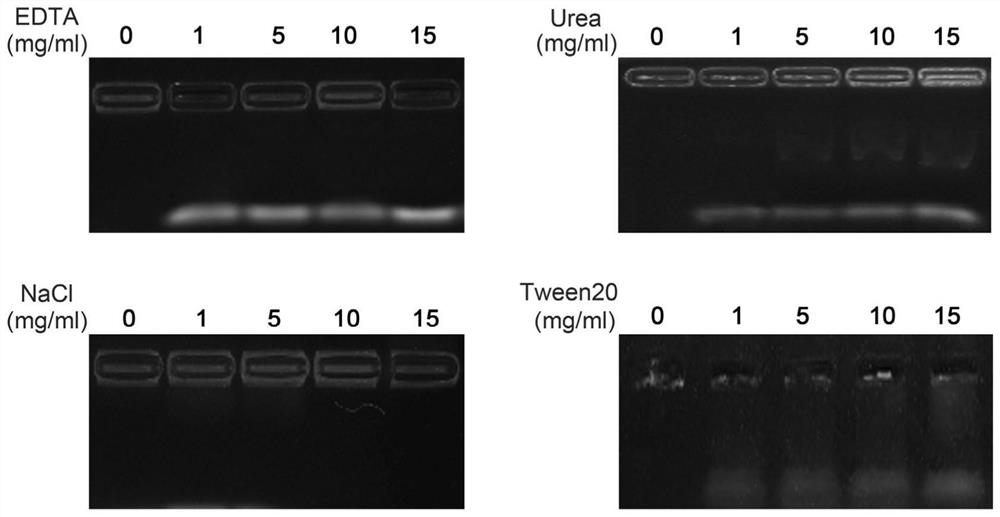
[0049] The specific results are as follows: the average particle diameter of the preparation can be controlled at 187±3.5nm, and the PDI is less than 0.3. The results of agarose gel electrophoresis figure 1 As shown, when Fe 3+ When the mo...
Embodiment 2
[0061] (1) Preparation of EGCG-Fe 3+ / siRNA / liposome
[0062] Soybean lecithin, cholesterol or cholesterol derivatives are dissolved in chloroform according to the prescription amount as the oil phase. EGCG-Fe 3+ / siRNA solution as the inner water phase W1. The inner water phase was added to the oil phase, and a W / O emulsion was formed by ultrasonication with a 300W probe under ice bath conditions. Add the W / O type emulsion dropwise to the external water phase containing the prescribed amount of egg yolk lecithin and DSPE-PEG2000, ultrasonicate in a 1000W water bath for 5-10 minutes to form a double emulsion, and evaporate under reduced pressure to remove the organic solvent to obtain EGCG-Fe 3+ / siRNA / liposome. At the same time, the particle size and polydispersity index (PDI) of the samples were measured using a Zetasizer 3000HS instrument particle size analyzer (Malvern Instruments, Malvern, UK).
[0063] The specific results are as follows: the average particle diamete...
Embodiment 3
[0078] (1) Preparation of TA-Fe 3+ / siRNA / biofilm complex
[0079] Suspend red blood cells extracted from whole blood in hypotonic buffer, sonicate for 5 minutes, collect cell membranes by centrifugation at low temperature, resuspend the collected red blood cell membranes in water at a concentration of 2 mg / ml, and mix with TA-Fe 3+ / siRNA / solution was mixed in equal volume, and the water bath was sonicated for 2 minutes. The nanoparticles are then collected by centrifugation at 4°C and 10000g for 5 minutes. The average particle diameter of the preparation is 140±2.5nm, and the PDI is less than 0.3.
[0080] (2) Preparation of TA-Fe 3+ / mRNA / biofilm complex
[0081] Platelet membranes were extracted from plasma by repeated freezing and thawing, suspended in water at a concentration of 2 mg / ml, and then the platelet membrane solution was mixed with TA-Fe 3+ / mRNA solution was mixed in equal volumes, incubated for 30 min, and then passed through 1000, 400, and 200 nm polyca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com