Amino acid as well as preparation method and application thereof
A technology of amino acids and carbamates, which is applied in the preparation of sulfonate esters, carboxylate esters, chemical instruments and methods, etc., and can solve the problems of cumbersome overall steps, low efficiency, and many overall steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention provides a kind of preparation method of amino acid, comprises the following steps:
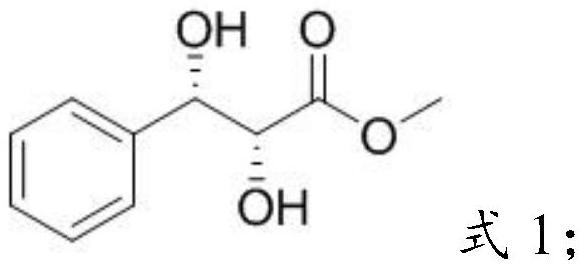
[0031] Mixing methyl cinnamate, AD-mix-alpha, methylsulfonamide and the first solvent, performing an asymmetric dihydroxylation reaction to obtain o-diol compounds;
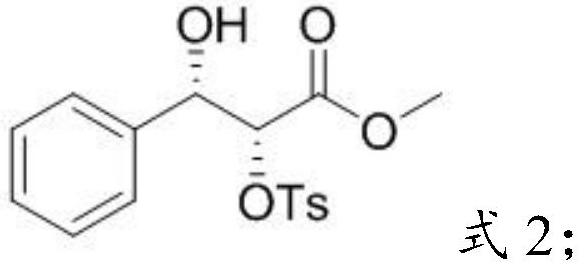
[0032] Mixing the o-diol compound, p-toluenesulfonyl chloride, triethylamine and a second solvent for esterification to obtain p-toluenesulfonate ester compounds;
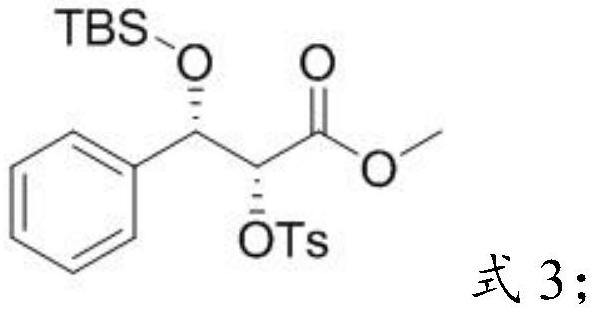
[0033] Mixing the p-toluenesulfonate ester compound, tert-butyldimethylsilyl chloride, 2,6-lutidine and a third solvent to carry out a silicon etherification reaction to obtain a first intermediate;
[0034] Mix the first intermediate, methyl-carbamate compound, sodium hydride and a fourth solvent to perform a substitution reaction to obtain a second intermediate; the methyl-carbamate compound is methyl-amino tert-butyl formate or benzyl methyl-carbamate;
[0035] mixing the second intermediate, the base and the fifth solvent, and perfo...
Embodiment 1
[0087] 1) Substitution reaction between methyl cinnamate C and AD-mix-alph to obtain o-diol compound D:
[0088]
[0089] Dissolve 3.65g (22.5mmol, 1eq) of methyl cinnamate in 110mL of tert-butanol and 110mL of water, then add 31g of AD-mix-alpha and 2.15g (22.5mmol, 1eq) of methylsulfonamide, under nitrogen protection, at 25°C The reaction was stirred for 24 hours; after the reaction was completed, sodium bisulfite solution was added to the resulting system to quench the reaction, extracted three times with ethyl acetate, the combined organic phases were washed with saturated brine, dried with anhydrous sodium sulfate, filtered, concentrated, column After separation by chromatography (PE:EA=2:1), the product was obtained as a white solid with a yield of 77%. 1 H NMR (400MHz, CDCl 3)δ7.54-7.31(m,5H),5.04(m,1H),4.39(s,1H),3.83(s,3H),3.16(s,1H),2.80(s,1H); MS(API -ES):[M+H] + = 197.2.
[0090] 2) Reaction of o-diol compound D with p-toluenesulfonyl chloride (TsCl) to obta...
Embodiment 2
[0104] Step 1~3) with embodiment 1;
[0105] 4) Compound F reacts with methyl-benzyl carbamate to prepare methyl ester compounds:
[0106]
[0107] 4.96g (30mmol) methyl-benzyl carbamate (CH 3 NHCbz) was dissolved in 30ml of anhydrous tetrahydrofuran and cooled to 0°C, NaH 1.32g (60% content, equivalent to 33mmol) was added in batches, and after stirring was continued at 0°C for 30 minutes, the resulting mixed solution was added dropwise to a solution containing 4.65g ( 10mmol) in anhydrous tetrahydrofuran (30mL) solution of compound F, and continue to cool down with an ice-water bath to ensure that the temperature of the reaction solution is 5°C; Adjust the pH to neutral with 1M dilute hydrochloric acid, concentrate the resulting mixture, disperse it in 200mL ethyl acetate, wash with 1M dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine successively, concentrate the obtained organic phase, and distill the concentrated The residue was sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com