Synthesis method of 3beta, 7beta (alpha) dihydroxy-5beta-cholanic acid
A technology of cholic acid and dihydroxy, which is applied in the field of organic chemical synthesis, can solve the problems of high price and achieve the effect of low price, less impurities and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
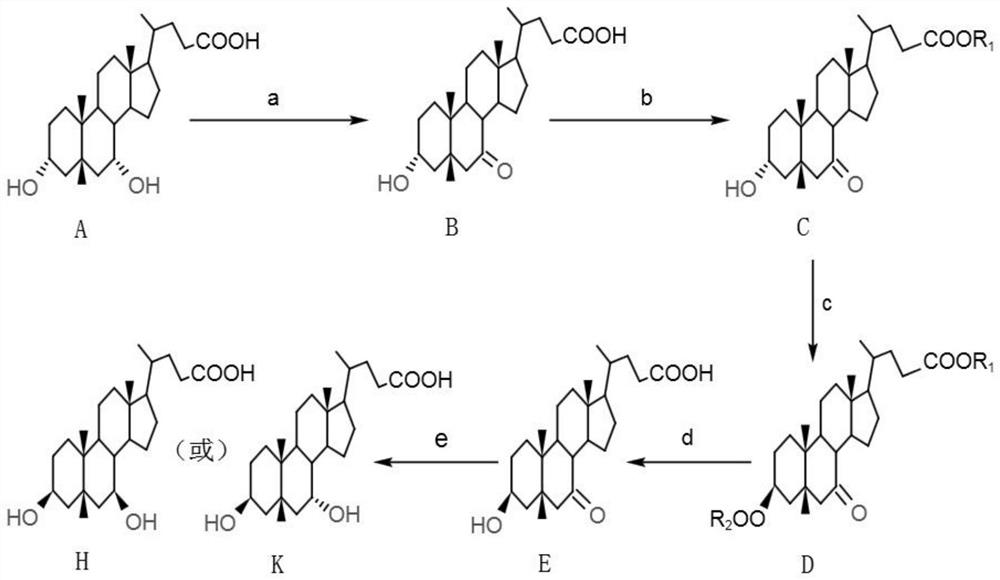
[0066] Such as figure 1 Shown, a kind of 3β, the synthetic method of 7β dihydroxy-5β-cholanic acid and 3β, 7α dihydroxy-5β-cholic acid, the starting material used is chenodeoxycholic acid, and concrete steps are as follows:
[0067] a, preparation of compound B (7-ketolithocholic acid)
[0068] Dissolving compound A (chenodeoxycholic acid) in acetone, lowering the temperature, adding an oxidizing agent to carry out oxidation reaction, after the reaction is complete, adding a reducing agent to terminate the reaction. Acetone was distilled off, filtered and washed to obtain the wet crude compound B. Compound B was suspended in 2 times the volume of methanol, heated to reflux for 2 hours, cooled to room temperature, filtered, washed and dried to obtain refined compound B.
[0069] b, preparation of compound C (7-ketolithocholate)
[0070] Compound B was dissolved in alcohol, catalyst was added, refluxed for 2 h, after the reaction was completed, sodium bicarbonate solution was...
Embodiment 1
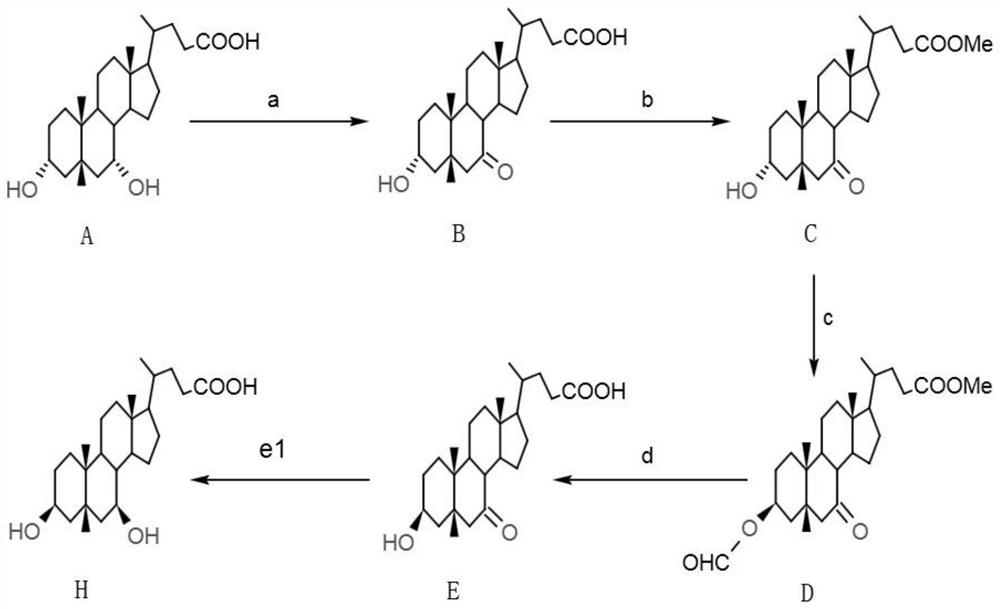
[0078] Such as figure 2 As shown, the preparation method of 3β, 7β-dihydroxy-5β-cholanic acid in this embodiment comprises the following steps:
[0079] a, preparation of 7-ketolithocholic acid
[0080] In a 500mL reaction flask, add 50g of chenodeoxycholic acid (CDCA), 250mL of acetone, stir to lower the temperature below 0°C, slowly add 22.3g of bromine, continue to react for 3 hours after the addition of bromine, and take samples to measure the residual amount of CDCA as 2.379%, add 1.3g sodium bisulfite to terminate the reaction. Acetone was distilled off in vacuo, 100 mL of water was added, stirred for 30 min, filtered and washed to obtain 84.7 g of wet crude product of 7-ketolithocholic acid. Pour all the wet crude 7-ketolithocholic acid into a reaction flask, add 170mL of methanol, raise the temperature and reflux for 2h, cool down to room temperature, filter, wash and dry to obtain 40.8g of 7-ketolithocholic acid with an HPLC purity of 99.347%.
[0081] b, preparat...
Embodiment 2
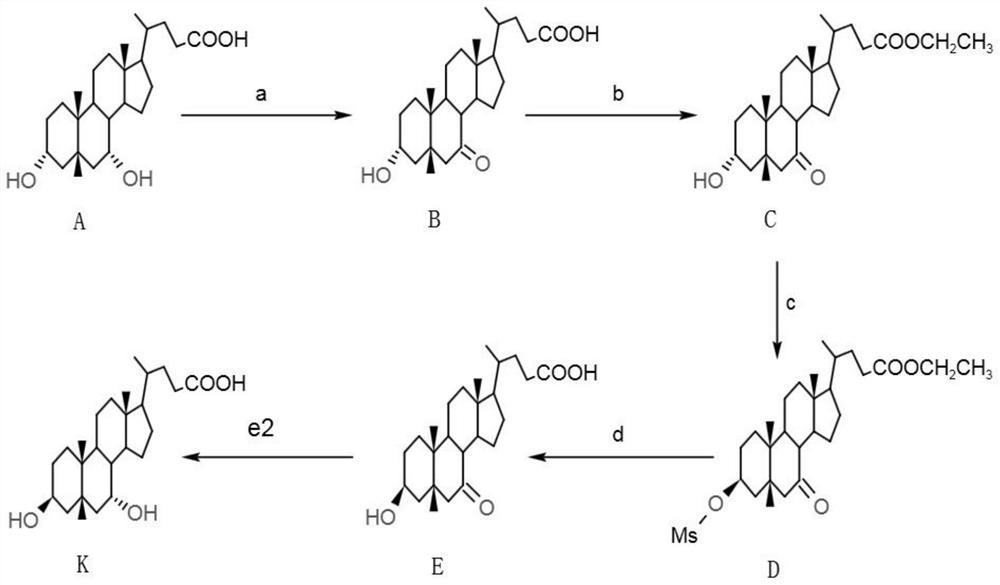
[0090] Such as image 3 As shown, the preparation method of 3β, 7α dihydroxy-5β-cholanic acid in this embodiment comprises the following steps:
[0091] a. Preparation of 7-ketolithocholic acid
[0092] In a 500mL reaction flask, add 50g of chenodeoxycholic acid (CDCA), 350mL of acetone, stir to lower the temperature below 0°C, add 0.5mL of concentrated sulfuric acid, slowly add 125g of 10% sodium hypochlorite solution, and continue the reaction for 0.5 h, the residual amount of CDCA was measured to be 3.446% by sampling, and 1 g of hydrosulfite was added to terminate the reaction. Acetone was distilled off in vacuo, 100 mL of water was added, stirred for 30 min, filtered and washed to obtain 82.3 g of wet crude product of 7-ketolithocholic acid. Pour the wet crude product of 7-ketolithocholic acid into a reaction flask, add 165mL of methanol, raise the temperature and reflux for 2h, cool down to normal temperature, filter, wash and dry to obtain 40.3g of 7-ketolithocholic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com