Copper oxide nanorod-heme functionalized graphene as well as preparation method and application thereof
A copper oxide nanorod, heme technology is applied in the direction of analysis by chemical reaction of materials, material analysis by observing the influence on chemical indicators, measurement devices, etc., which can solve the problem of low catalytic performance and aggregation catalytic efficiency. , difficult to disperse and other problems, to achieve the effect of high catalytic performance, improved material dispersibility, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
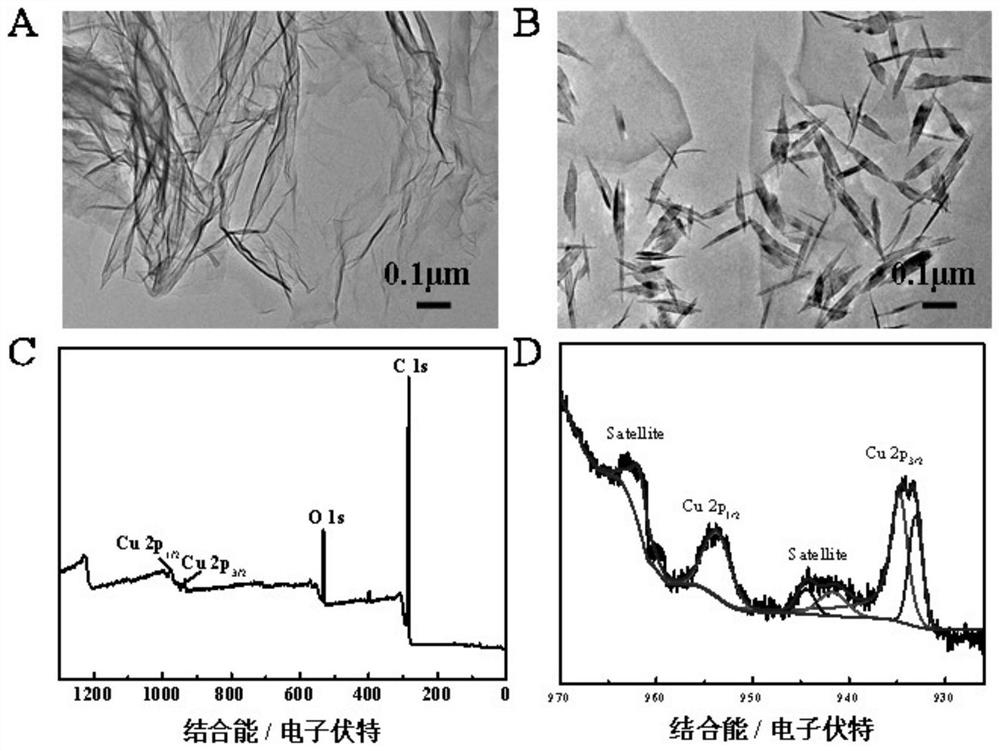
Image
Examples
Embodiment 1
[0027] Embodiment 1 prepares CuO / H-Gr, specific steps:
[0028] (1) GO was synthesized according to the Hummers method, and the prepared 5.0 mg GO was ultrasonically dispersed in 10 mL of secondary water to obtain 0.50 mg·mL -1 GO suspension;
[0029] (2) Dissolve 7.0 mg hemin in the GO suspension prepared in step (1), stir for 10 min, and add 200 μL NH 3 ·H 2 O and 80 μL N 2 h 4 ·H 2 O(NH 3 ·H 2 O and N 2 h 4 ·H 2 The mass fractions of O are 25% and 80% respectively); in a water bath at 60°C for 3.5h, let stand to cool to room temperature; centrifuge at 12000rpm for 15min; the product obtained is washed twice with secondary water, and then prepared by adding secondary water into 0.50mg·mL -1 H-Gr suspension;
[0030] (3) 200μL 0.020M Cu(NO 3 ) 2 ·3H 2 O and 100μL 2.0mg·mL -1 Add PVP to 2.0mL of the H-Gr suspension prepared in step (2), stir magnetically for 15min; under vigorous stirring, heat the mixture to 100°C, then quickly add 1.0mL 0.50M NaOH solution, c...
Embodiment 2
[0032] Example 2 The verification that CuO / H-Gr has peroxidase-like activity, specifically:
[0033] In 1590μL 0.20M HAc-NaAc buffer solution (pH 4.0), add 10μL 0.50mg·mL -1CuO / H-Gr obtained in Example 1 above; then add 200 μL 10 mM TMB and 200 μL 100 mM H 2 o 2 , produce a blue product oxTMB; measure the absorbance at λ=652nm with a UV-Vis absorption spectrophotometer.
[0034] Control experiment 1: replace CuO / H-Gr in the reaction system of Example 2 with H-Gr, and the rest of the experimental system is the same as that of Example 2.
[0035] Control Experiment 2: CuO / H-Gr in the reaction system of Example 2 was replaced by CuO, and the rest of the experimental system was the same as that of Example 2.
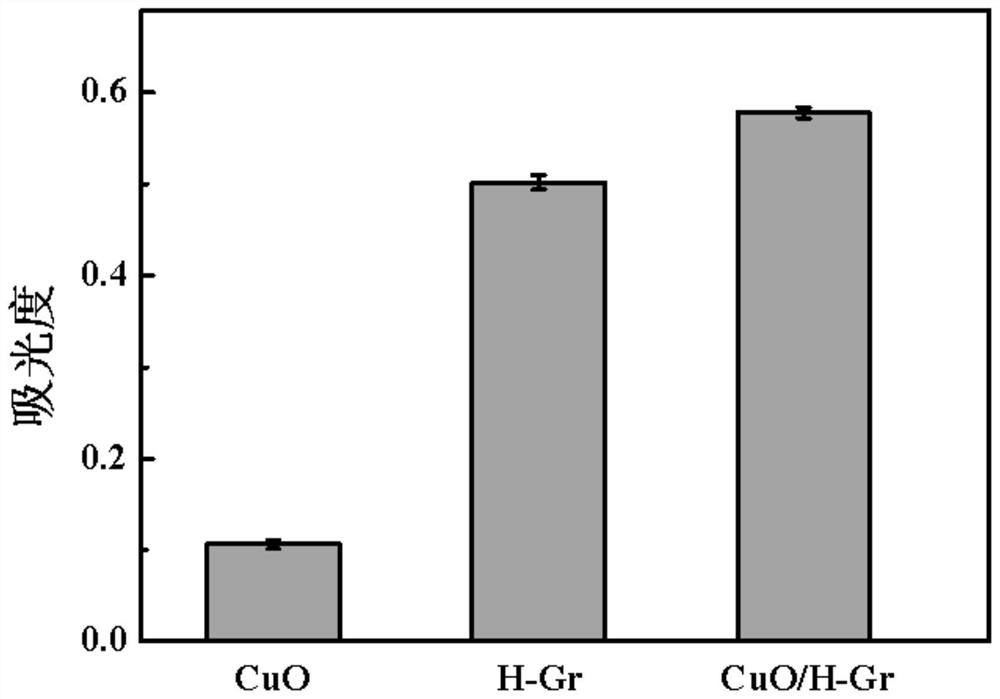
[0036] Such as figure 2 As shown, the absorbance of Example 2 is higher than that of Control Experiment 1 and Control Experiment 2, indicating that CuO / H-Gr has a synergistic catalytic effect.
Embodiment 3
[0037] Example 3 CuO / H-Gr acts as a peroxidase-like catalyst for H 2 o 2 The application experiment of oxidation substrate, the specific steps are as follows:
[0038] In 1590μL 0.20M HAc-NaAc buffer solution (pH 4.0), add 10μL 0.50mg·mL -1 CuO / H-Gr obtained in Example 1 above; then add 200 μL 12 mM TMB and 200 μL 120 mM H 2 o 2 , produce blue product oxTMB; observe the reaction color change.
[0039] Control Experiment 1: The TMB in the reaction system of Example 3 was replaced by o-phenylenediamine (OPD), and the rest of the experimental system was the same as that of Example 3.
[0040] Control Experiment 2: TMB in the reaction system of Example 3 was replaced by 2,2-azino-bis(3-ethyl-benzothiazole-6-sulfonic acid) (ABTS), and the rest of the experimental system was the same as that of Example 3.
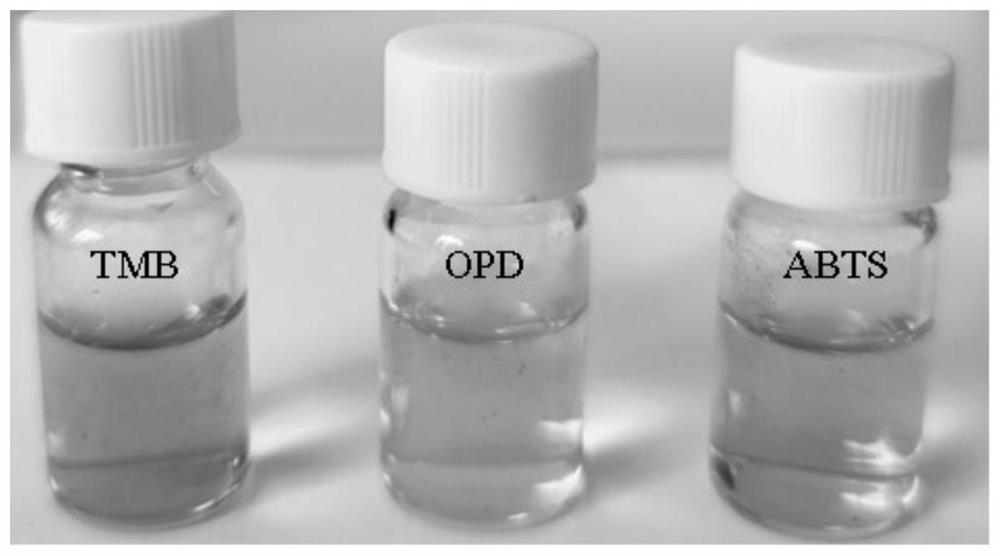
[0041] Such as image 3 shown in H 2 o 2 In the presence of CuO / H-Gr, TMB, OPD and ABTS can be catalyzed to oxidize to blue, yellow and green products, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com