Method for preparing ammonium chloride through co-production of baking soda
A baking soda and ammonium chloride technology, which is applied in the direction of ammonium chloride, ammonium halide, carbonate preparations, etc., can solve the problems of high ammonium salt content, reduced filtration effect, excessive ammonium salt content, etc., and achieve the reduction of ammonium salt content, reduce cleaning difficulty, and reduce the effect of ammonium salt content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
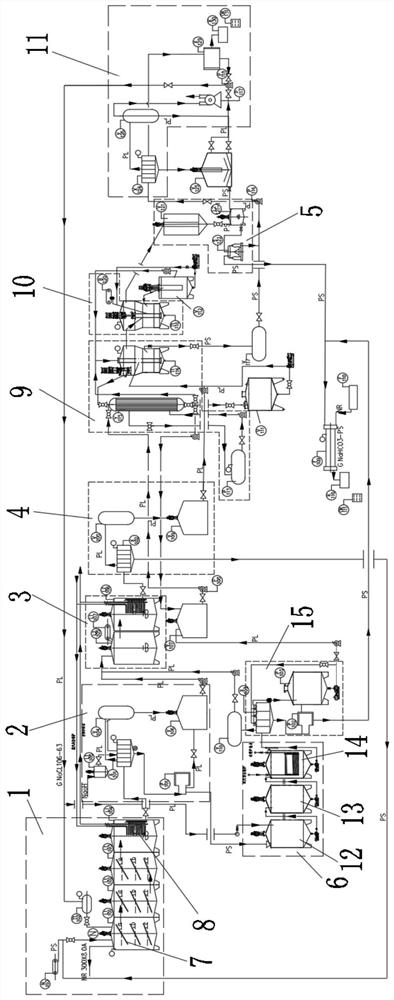
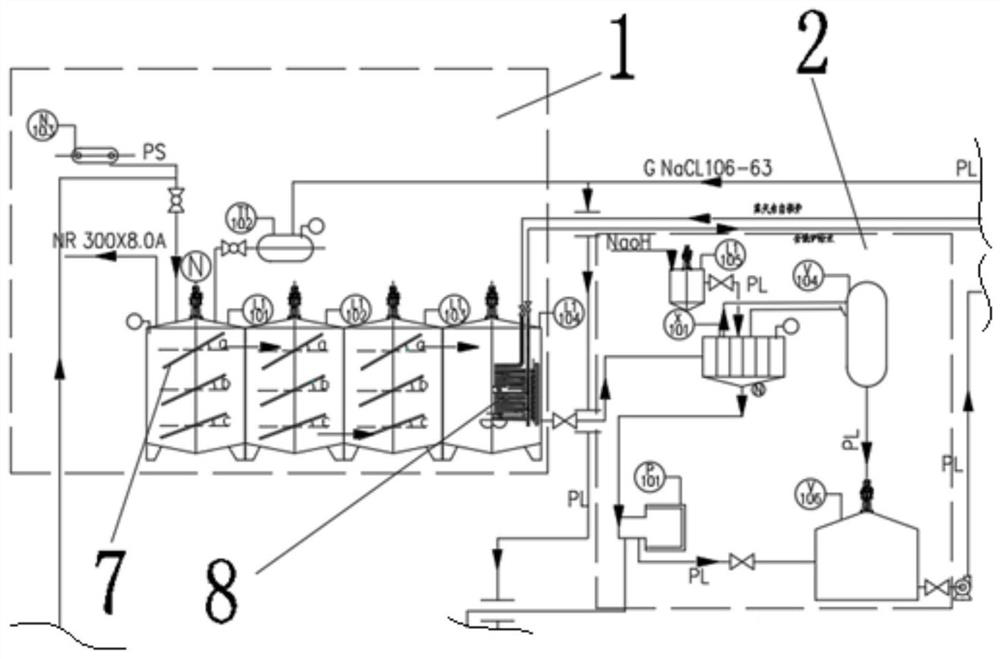
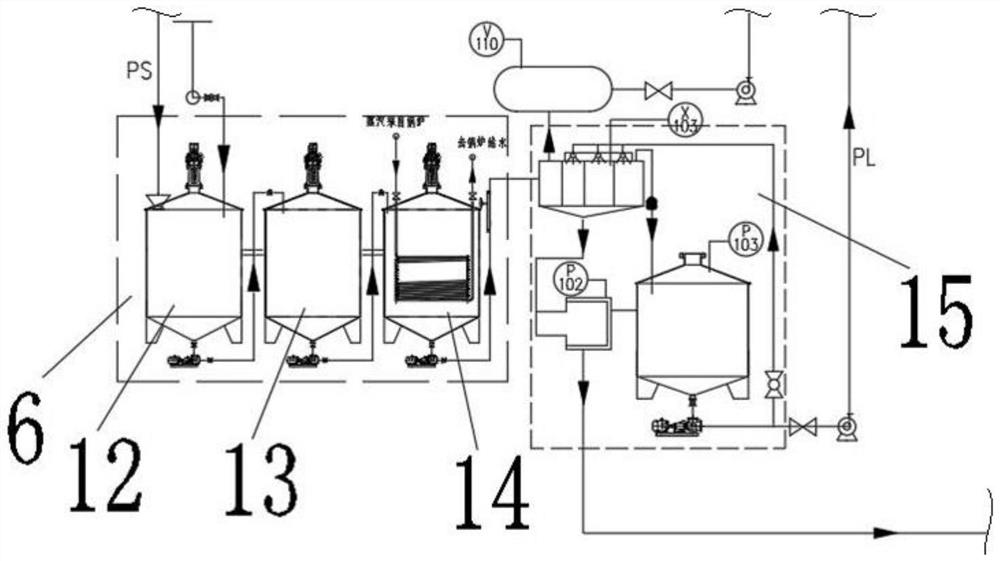
[0037] A kind of method that baking soda co-produces ammonium chloride prepares the baking soda of low ammonium salt content, comprises the following steps:
[0038] 1) Primary precipitation of baking soda: directly add 8.5 cubic meters of unpurified saturated brine to the mixed reaction kettle L1101 with a capacity of 11 cubic meters through the brine tank T1102, raise the temperature to 35°C, and then pass through the ammonium bicarbonate feeding device N103 ammonium bicarbonate The metering belt adds 2,200 kg of ammonium bicarbonate to the mixing reactor L1101, and under the action of stirring, the temperature is controlled at 25°C for a metathesis reaction; when the mixing reactor L1101 is about to be full, open the valve at the bottom of the mixing reactor L1101, start the pump, Send the slurry into the crystal nucleus growth kettle L1102, continue stirring, and control the temperature at 28°C; when the crystal nucleus growth kettle L1102 is about to be full, open the valv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com