Collecting agent with carboxyl-hydroximido structure, preparation of collecting agent and application of collecting agent in flotation
A collector and hydroxime technology, applied in flotation, solid separation, etc., can solve the problems of difficulty in preparing combined agents, temperature sensitivity, poor water solubility, etc., and achieve the effects of improving flotation efficiency, enhancing adsorption, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
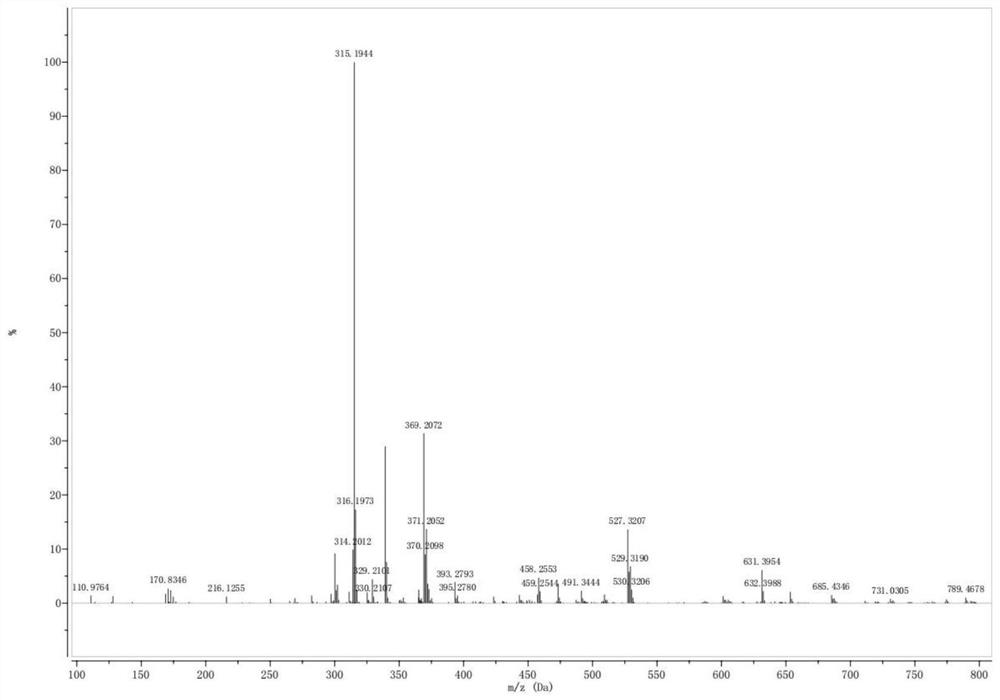
[0072] Mix 14.86g of L-glutamic acid (content: 99%) and 20mL of methanol, then add 2g of concentrated sulfuric acid catalyst, heat to 70°C, stir and reflux for 5h to obtain L-glutamic acid-1-methyl ester. Add 17.58g of n-decanoic acid (98% content) and 16.72g N,N'-carbonyldiimidazole (CDI) (97% content) into the ball mill jar, grind for 30min, then add the L-glutamic acid-1 obtained above - Methyl ester continued grinding for 1 h. Then add 6.98g hydroxylamine hydrochloride (content is 98.5%) and 8.33g sodium hydroxide (content is 96%), continue to grind for 30min, add water to dissolve, and adjust pH to about 5 with 3mol / L hydrochloric acid, leave standstill to wait for floc After precipitation, the orange-yellow solid product was obtained after cooling and filtration, which was the target collector product. After analyzing and detecting, the content of collector product 4-decanoylamino-4-hydroxycarbamoyl butyric acid is 49.95%, based on the yield of 4-decanoylamino-4-hydroxy...
Embodiment 2
[0075] Mix 13.31g of L-aspartic acid (100% content) and 20mL of methanol, then add 2g of concentrated sulfuric acid catalyst, heat to 70°C, stir and reflux for 5h to obtain the corresponding L-aspartic acid-1-methyl ester. Add 17.58g of n-decanoic acid (98% content) and 16.72g N,N'-carbonyldiimidazole (CDI) (97% content) into the ball mill jar, grind for 30min, then add the L-aspartic acid obtained above- 1-Methyl ester, continue grinding for 1h. Then add 6.98g hydroxylamine hydrochloride (content is 98.5%) and 8.33g sodium hydroxide (content is 96%), continue to grind for 30min, add water to dissolve, and adjust pH to about 5 with 3mol / L hydrochloric acid, leave standstill to wait for floc After precipitation, the orange-yellow solid product was obtained after cooling and filtration, which was the target collector product.
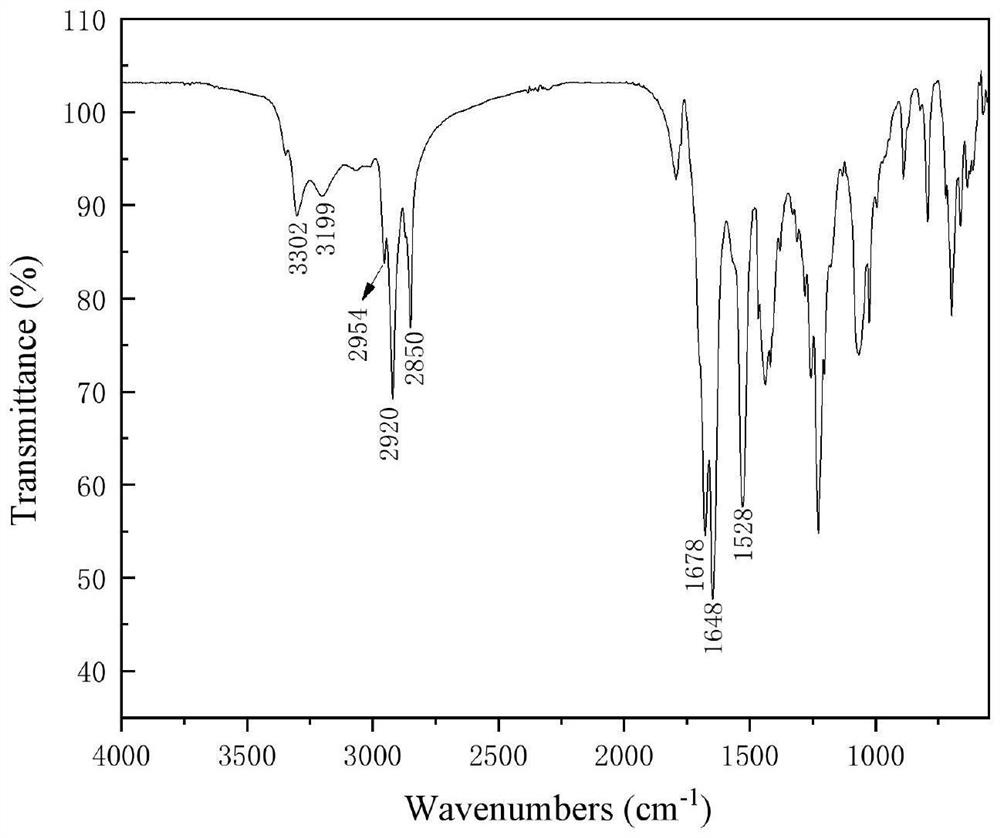
[0076] The product is characterized after separation and purification by column chromatography, and the infrared spectrum of 3-decanoylamino-3-hydroxyca...
Embodiment 3
[0079] Mix 14.86g L-glutamic acid (99% content) and 20mL methanol, then add 2g concentrated sulfuric acid catalyst, heat to 70°C, stir and reflux for 5h to obtain L-glutamic acid-1-methyl ester. Add 20.44g of lauric acid (98% content) and 22.70g N,N'-dicyclohexylcarbodiimide (DCC) (99% content) into a ball mill, grind for 30min, then add the L-glutamine obtained above Acid-1-methyl ester was further triturated for 1 h. Then add 6.98g hydroxylamine hydrochloride (content is 98.5%) and 8.33g sodium hydroxide (content is 96%), continue to grind for 30min, add water to dissolve, and adjust pH with 3mol / L hydrochloric acid to be about 5, leave standstill to treat floc After precipitation, the orange-yellow solid product was obtained after cooling and filtration, which was the target collector product.
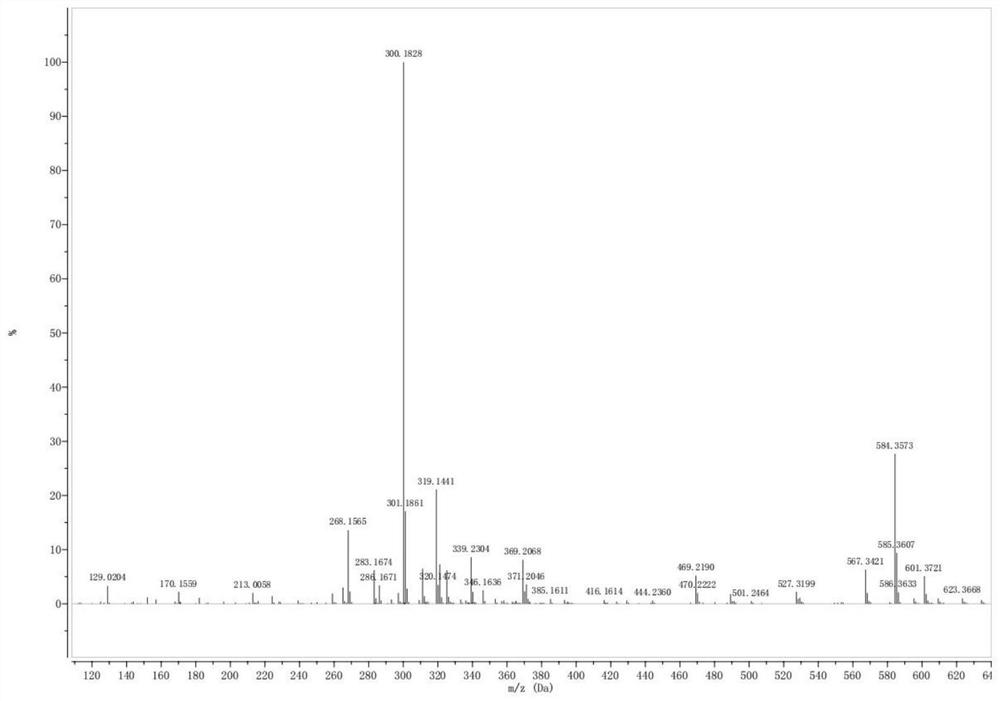
[0080] The mass spectrum of 4-dodecanoylamino-4-hydroxycarbamoylbutanoic acid is as Figure 4 As shown, the peak with a mass-to-charge ratio of 343.2214 in the spectrum is [M-H], ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com