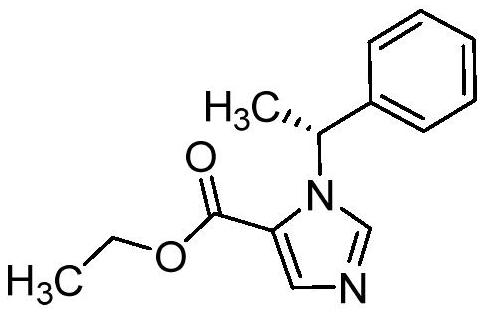
Method for purifying etomidate
A technology of etomidate and purification method, applied in the direction of organic chemistry and the like, can solve the problems of increased production cost, decreased process yield and high impurity content, and achieves the effects of low production cost, high process yield and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
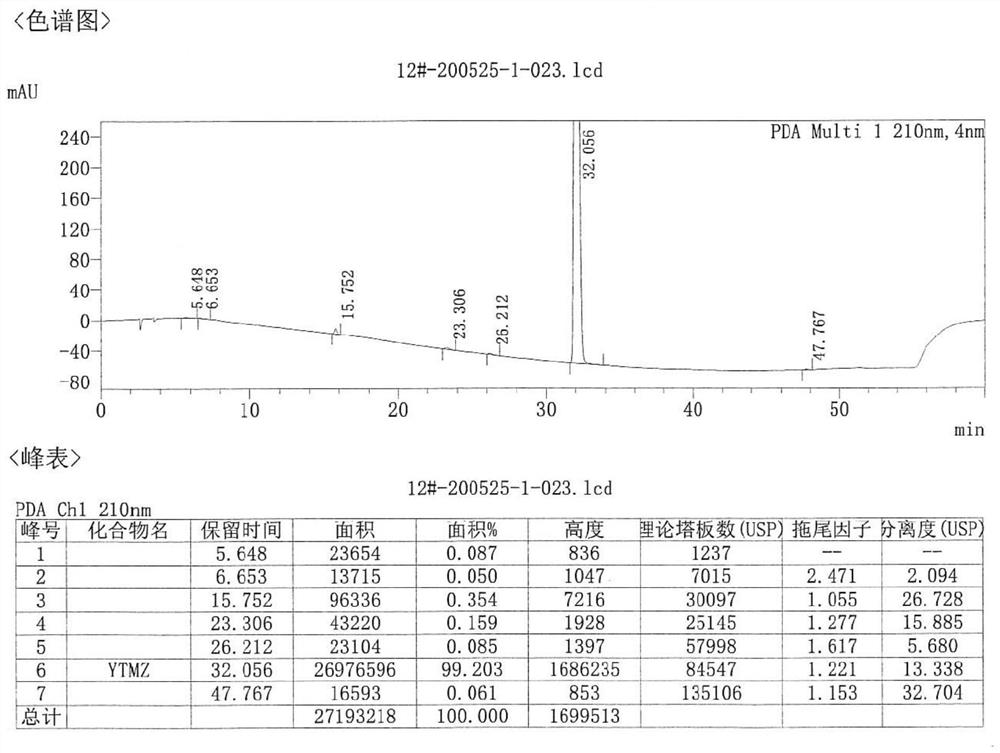
[0043] The object that present embodiment handles is etomidate crude product (HPLC purity 97.93%, maximum simple and complex 0.78%), and it is prepared by the following steps, specifically:
[0044] a) Take 60g of R-(+)-α-methylbenzylamine, 50g of triethylamine, 65g of ethyl chloroacetate, and 100mL of toluene, add them into the reaction bottle, start stirring; react at about 70°C for 8 hours. Cool down, filter, and wash the filtrate three times with water.
[0045] b) Add the filtrate after washing with water into the reaction flask, add 67g of formic acid, reflux and divide the water for 3 hours. The temperature was lowered, the reaction solution was washed three times with water, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a yellow liquid.
[0046] c) Add the yellow liquid obtained in step b) into a reaction flask, add 500 mL of toluene, 35 g of sodium ethoxide, and 100 g of ethyl formate. Reaction at room temperature ...
Embodiment 2
[0064] The refining process of etomidate crude product in the present embodiment is as follows:
[0065] Step 1) Add 10 g of crude etomidate, 20 mL of a mixed solvent of DMSO and water into the reaction bottle, start stirring, raise the temperature to about 70 ° C, stir and dissolve to obtain a solution, and the volume ratio of DMSO and water in the mixed solvent is 1:2 .
[0066] Step 2) Cool down the solution obtained in step 1) to about 5° C., and keep it warm for 1 hour for crystallization. Filtrate, wash, and dry to obtain 8.6 g of etomidate as a white solid, with a yield of 86%.
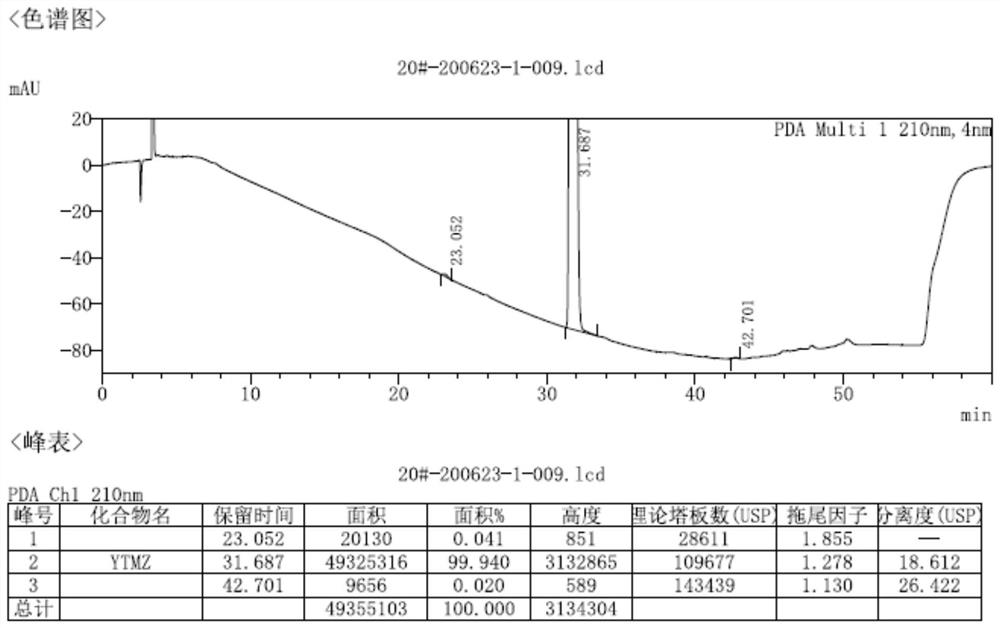
[0067] Using the same detection conditions as in Example 1 to detect the related substances of the product, the HPLC purity was 99.86%, and the maximum simple impurity was 0.05%.
Embodiment 3
[0069] The refining process of etomidate crude product in the present embodiment is as follows:
[0070] Step 1) Add 10 g of crude etomidate, 15 mL of a mixed solvent of N,N-dimethylformamide and water into the reaction bottle, start stirring, raise the temperature to about 70°C, stir and dissolve to obtain a solution, the N,N- The volume ratio of dimethylformamide and water is 4:3.
[0071] Step 2) Cooling the solution obtained in step 1) to about 45° C., adding seed crystals (crude etomidate) in an amount of 0.5% of the weight of the crude etomidate. The temperature was lowered to about 10°C, and the crystallization was carried out by heat preservation for 1 hour. Filtration, washing, and drying yielded 8.5 g of etomidate in the form of a white solid. Yield 85%.
[0072] Using the same detection conditions as in Example 1 to detect the related substances of the product, the HPLC purity was 99.92%, and the maximum simple impurity was 0.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com