Preparation method of 19-nor-4-androstenedione
A technology of androstenedione and compound is applied in the field of preparation of 19-nor-4-androstenedione, can solve the problems of low yield, many impurities, difficult to purify into white, etc., achieves improved yield and Purity, low refining cost, easy decolorization and refining effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
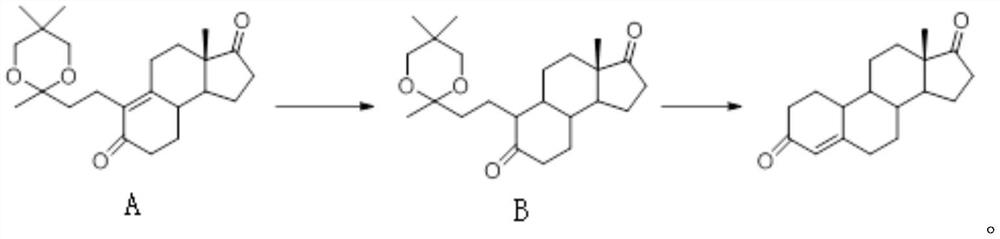
[0025] 100g of compound A, 1000g of ethanol, and 3g of pyridine were stirred and dissolved, and put into a high-pressure hydrogenation kettle. After the kettle was replaced with nitrogen three times, 3g of palladium carbon was dropped into the kettle (the weight content of palladium was 5%). Open the vacuum valve, evacuate to the pressure in the autoclave ≤ -0.08Mpa, open the hydrogen to positive pressure; repeat the operation again, open the hydrogen valve, and pressurize the autoclave to 0.1MPa. The temperature in the kettle was raised to 60°C for reaction, and after the temperature of the system became stable, the pressure of the reaction kettle was increased again and maintained to 0.3MPa. After the reaction of the raw materials was completed, the temperature of the reaction system was lowered to room temperature 25°C, the pressure of the reaction kettle was first slowly released to normal pressure, and then the system was replaced with nitrogen for 15 minutes. The system...
Embodiment 2
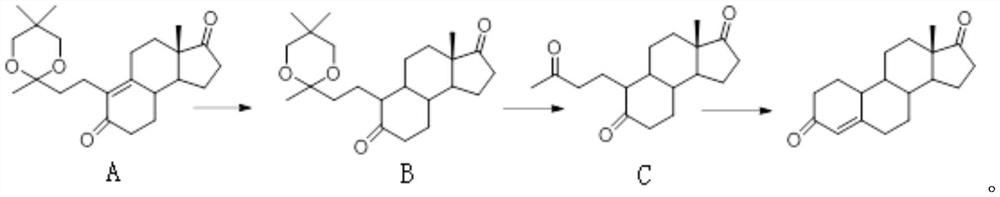
[0030] The preparation method of Compound C wet product is the same as in Example 1.
[0031] Add 50g of isopropanol and 150g of water to the wet product of compound C, then add 80g of sodium bisulfate solution (weight concentration: 30%), the system is heated up to 80°C to form a ring reaction, after the reaction is complete, cool down to 60°C, and slowly add 1.5kg Water analysis was carried out with tap water, the temperature of the system was lowered to 10° C., filtered, and washed with water to obtain crude 19-nor-4-androstenedione (acidic decarboxylation product). The crude product was dissolved in 600 g of methanol and 5 g of activated carbon, decolorized and recrystallized to obtain the refined product of 19-nor-4-androstenedione (acidic decarboxylation product). The total yield is 64%, the purity is 99.4%, and the product color is white.
Embodiment 3
[0033] The preparation method of the ethanol solution of Compound B is the same as in Example 1.
[0034] The ethanol solution of Compound B was concentrated under negative pressure at 50°C (vacuum ≤ -0.08Mpa), replaced once with 100 g of water, and concentrated again until no distillate was present to obtain the wet product of Compound B.
[0035] Add 300g of isopropanol to the wet product of compound B, then add 45ml of concentrated hydrochloric acid, the system is heated up to 50°C to form a cyclic reaction, after the reaction is completed, 1.8kg of tap water is slowly added for water analysis, the system is cooled to 10°C, filtered, and washed with water to obtain 19- Nor-4-androstenedione (acid decarboxylation) crude product. The crude product was dissolved in 600 g of ethanol and 5 g of activated carbon, decolorized and recrystallized to obtain the refined product of 19-nor-4-androstenedione (acidic decarboxylation product). The total yield is 63%, the purity is 99.3%, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com