Recombinant alginate lyase AlyL7 and application thereof
A technology of alginate lyase and alginate oligosaccharide, applied in the direction of lyase, carbon-oxygen lyase, recombinant DNA technology, etc., can solve the problems of alginate oligosaccharide difficulty, low enzyme production of wild-type strains, etc., to overcome the catalytic Low efficiency, good application prospects, and the effect of improving plant stress resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Source and sequence analysis of alginate lyase AlyL7
[0023] The Flavobacterium used in this example was provided by Fuzhou University.
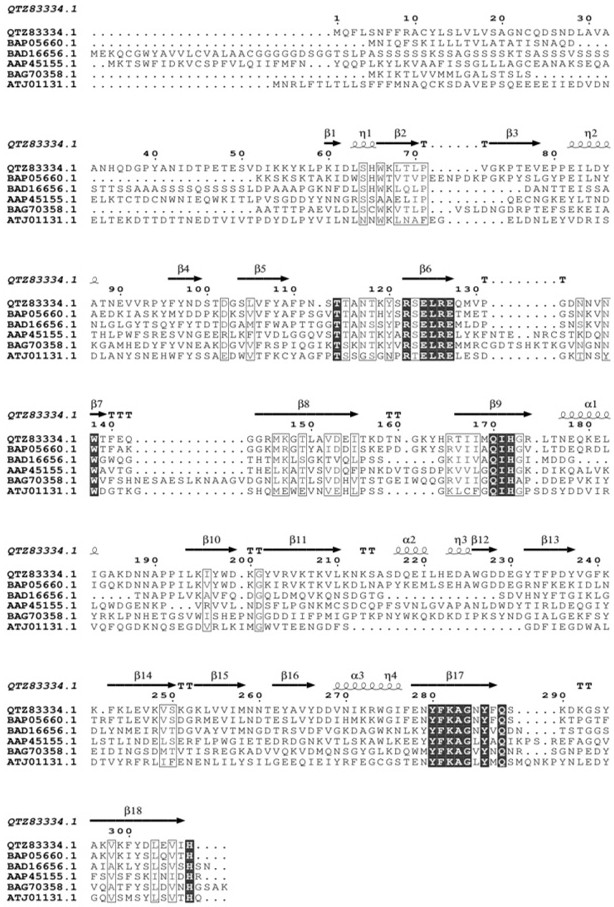
[0024] Alginate lyase of the present invention alyl7 Flavobacterium Zobellia sp. .The genome is amplified by means of chromosome walking. This sequence contains 924 bases (as shown in SEQ ID NO.2), which encodes 307 amino acids (as shown in SEQ ID NO.1). Upload the base sequence Until NCBI obtained the sequence accession number MW561203.1, the homology comparison results showed that the similarity between it and the existing alginate lyase CaAly1 (ADV51457.1) nucleotide sequence was only 63.4%. According to the results of multiple sequence alignment, the amino acid sequence of recombinant alginate lyase AlyL7 includes the conserved sequence R(S / N)E(L / V)R, QIH, YFKAG(V / I)Y(N / P)O( figure 1 ), so the alginate lyase AlyL7 belongs to the 7th family of polysaccharide hydrolases (PL7).
Embodiment 2
[0025] Example 2: Construction of engineering bacteria for recombinant expression of alginate lyase AlyL7
[0026] With BamH Ⅰ and Xho Ⅰ as restriction sites, primers were designed so that the obtained alyl7 Restriction sites are placed at both ends of the gene sequence.
[0027] Forward primer AlyL7F (SEQ ID NO.3):
[0028] 5'-CG CGGATC CATGCAGTTTTTAAGCAACT-3' (the underline is the BamH Ⅰ restriction site)
[0029] Reverse primer AlyL7R (SEQ ID NO.4):
[0030] 5'-CC GCTCGA GGTGTATAACTTCTAAATCGT-3' (Xho Ⅰ restriction site is underlined)
[0031] The PCR reaction was carried out under the following conditions: pre-denaturation at 94 °C for 5 min; denaturation at 94 °C for 30 s; annealing at 55 °C for 20 s; extension at 72 °C for 2 min, for a total of 30 cycles, followed by extension at 72 °C for 10 min. The PCR product was gel-cut and recovered after 1% agarose nucleic acid electrophoresis to obtain the target gene with restriction site, and the target gene with restric...
Embodiment 3
[0032] Example 3: Induced fermentation of recombinant alginate lyase AlyL7
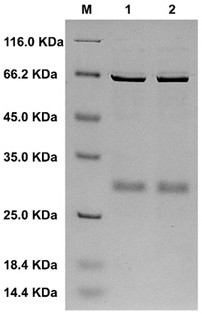
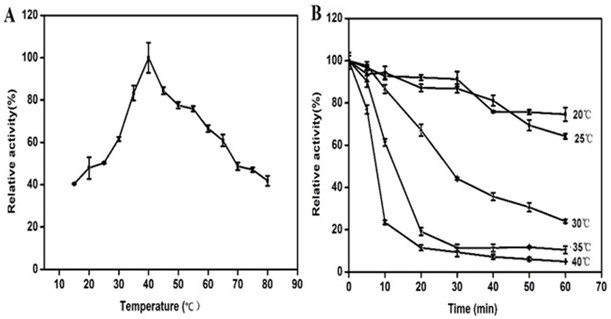
[0033] recombinant bacteria E.coil BL21(DE3)-pGEX-4T-1- alyl7 in the presence of 100 μg / mL Amp + Three-section line was performed on the LB solid medium plate, and a single colony was picked to 5 mL containing 100 μg / mL Amp + In LB liquid medium, cultivate overnight at 37°C in a shaker to obtain seed liquid. Inoculate the seed solution to 1 L containing 100 μg / mL Amp at an inoculum volume of 1 vol%. + LB liquid medium, cultured to OD 600When it is 0.6-0.8, add isopropylthiogalactopyranoside (IPTG) at a final concentration of 0.8 mM to the medium, and induce at 20 °C for 18 h. Centrifuge at 12,000 rpm at 4°C for 15 min to collect the bacteria, and use pre-cooled 40 mL buffer A (140 mM NaCl, 2.7 mM KCl, 10 mM NaCl 2 HPO 4 , 1.8 mM KH 2 PO 4 ; pH 7.4) for resuspension, ultrasonication for 30 min, and centrifugation at 12,000 rpm at 4°C for 15 min to collect the supernatant, which is the crude e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com