Vanadium pentoxide as well as ammonium-free preparation method and application thereof
A technology of vanadium pentoxide and vanadium oxysulfate, which is applied to the preparation of vanadium oxide and vanadium compounds, and the improvement of process efficiency. It can solve the problems of high production cost, low production cost, and large specific surface area, and achieve cost reduction and Effects of environmental pollution, reduction of emissions, and simplification of production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
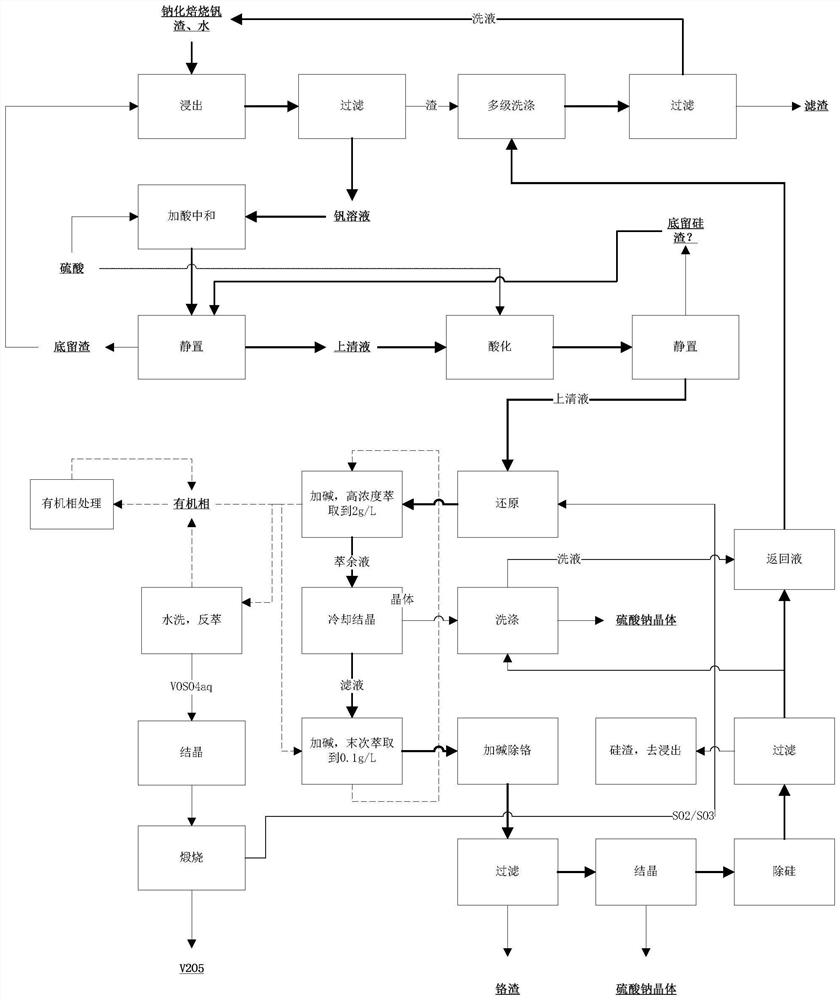
[0047] This embodiment discloses a method for preparing vanadium pentoxide without ammonium, such as figure 1 shown, including the following steps:
[0048] 1. The ratio of sodium carbonate to vanadium slag (V 8.3%) is 1:1, calcined at 850°C for 12 hours in an air atmosphere, and the calcined product is clinker;
[0049] 2. Leach the clinker with water according to the liquid-solid ratio of 4:1, and obtain the vanadium slag leach solution containing V 31g / L;
[0050] 3. Add sulfuric acid to acidify until the pH value is 2, maintain the temperature at 80°C, keep stirring, and introduce sulfur dioxide until the solution turns pure blue;
[0051] 4. Use P 2 0 4 As the extraction agent, sodium hydroxide is used to adjust the pH value, and the vanadium is extracted to the organic phase; dilute sulfuric acid is used as the stripping agent, and the extraction is repeated until the vanadium concentration reaches 50g / L;
[0052] 5. Evaporation and crystallization obtains vanadyl su...
Embodiment 2
[0056] This embodiment discloses a method for preparing vanadium pentoxide without ammonium, comprising the following steps:
[0057] 1. The ratio of sodium carbonate to petroleum fly ash (V 16.6%) is 2:1, and it is calcined at 900°C for 6 hours in an air atmosphere, and the calcined product is clinker;
[0058] 2. Leach the clinker with water according to the liquid-solid ratio of 4:1, and obtain the vanadium slag leach solution containing V 40g / L;
[0059] 3. Add sulfuric acid to acidify until the pH value is 1.5, maintain the temperature at 90°C, and continue to stir. According to the amount of vanadium, add citric acid of 1 / 16 the amount of vanadium, and continue to stir until the solution turns pure blue;
[0060] 4. Use P 2 0 4 As the extraction agent, sodium hydroxide is used to adjust the pH value, and the vanadium is extracted to the organic phase; dilute sulfuric acid is used as the stripping agent, and the extraction is repeated until the vanadium concentration reac...
Embodiment 3
[0064] This embodiment discloses a method for preparing vanadium pentoxide without ammonium, comprising the following steps:
[0065] 1. The ratio of sodium carbonate to petroleum gasification coke residue (V 3.2%) is 5:1, calcined at 700°C for 24 hours in an air atmosphere, and the calcined product is clinker;
[0066] 2. Leach the clinker with water according to the liquid-solid ratio of 2:1, and obtain the vanadium slag leach solution containing V 70g / L;
[0067] 3. Add sulfuric acid to acidify until the pH value is 2, maintain the temperature at 90°C, and continue to stir. According to the amount of vanadium, add zinc powder, continue to stir until the solution turns pure blue, and filter out the residual zinc powder;
[0068] 4. Use P 2 0 4 As the extraction agent, sodium hydroxide is used to adjust the pH value, and the vanadium is extracted to the organic phase; dilute sulfuric acid is used as the stripping agent, and the extraction is repeated until the vanadium conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com