Preparation and application of photo-response nitric oxide delivery/photo-thermal synergistic material
A nitric oxide, photothermal synergistic technology, applied in medical preparations with non-active ingredients, wave energy or particle radiation treatment materials, medical preparations containing active ingredients, etc., can solve the problems of biological damage and achieve improved Stability, excellent photothermal effect, and synergistic therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
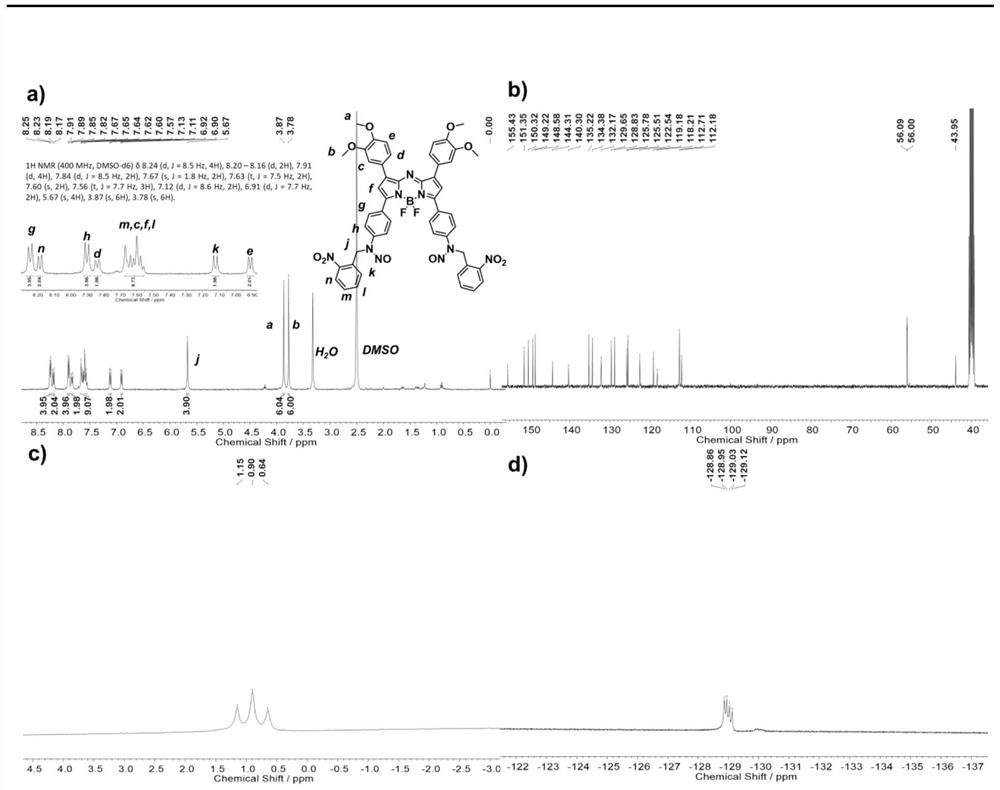
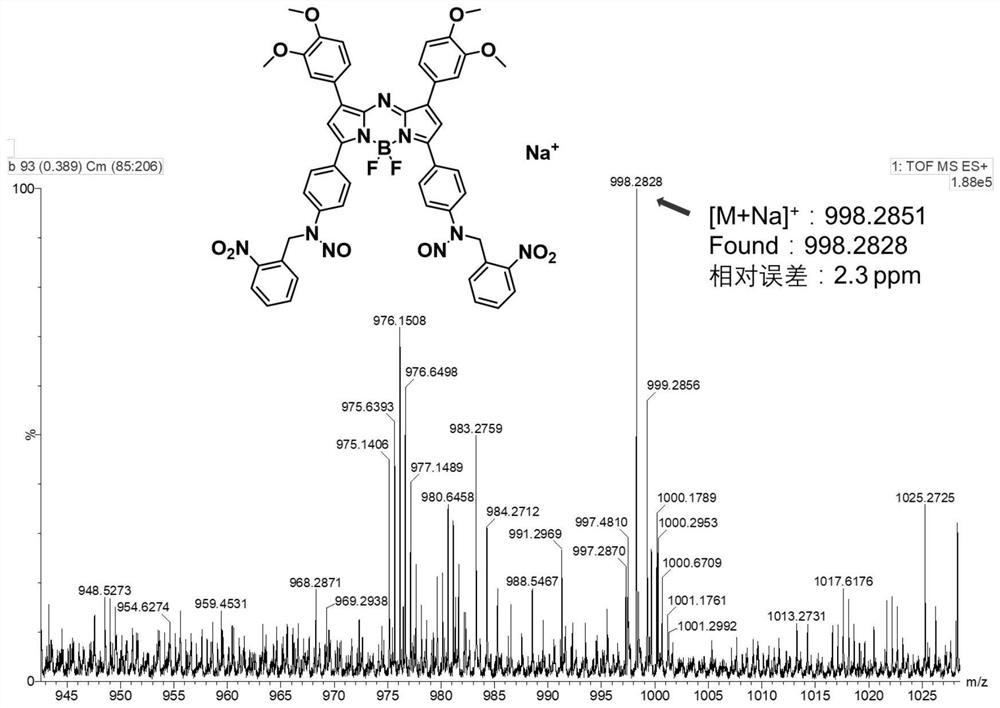
[0047] In the first step, the nitric oxide releasing unit is prepared, the Schiff base is generated by the reaction of the aldehyde group and the amino group, then the Schiff base is reduced, and finally the nitric oxide releasing unit is obtained through the nitrosation reaction. The synthesis process is simple and easy to operate. In order to help understand more clearly, one of the structural compounds of formula I is mainly selected as an example below, and the specific reaction formula is as follows:
[0048]
[0049] Nitric oxide donor preparation method:
[0050] A (250mg, 0.381mmol, 1eq) and o-nitrobenzaldehyde (1.174g, 7.722mmol, 20eq) were added into a mixed solvent of absolute ethanol and anhydrous THF (1:1, 30mL), and refluxed at 90°C. After reacting for 24 hours, the solvent was removed by rotary evaporation, and ethanol was added for ultrasonic washing, suction filtered, washed with ethanol and dried to obtain 220 mg of dark blue powder B (71.8%).
[0051] B...
Embodiment 2
[0058] Example 2: White light triggers the release of nitric oxide and 808nm laser light to produce photothermal effect
[0059] Determination of NP-1, NP-2 nitric oxide release by UV-Vis absorption spectroscopy: using white LED light (62.6mW / cm 2 ) According to the nanoparticle assembly NP-1 or NP-2 obtained above, track the change of its ultraviolet-visible light absorption spectrum. The test results show that: with the prolongation of the illumination time, the absorption peak of polymer nanoparticles at 711nm decreases rapidly, and the corresponding new absorption peak at 780nm increases rapidly, and finally tends to balance, indicating that the polymer nanoparticles have photoresponsiveness. And after adding the Giress reagent, the color of the solution turns purple, indicating that nitric oxide is released, and the apparent color of the polymer nanoparticles changes from the initial blue to purple when illuminated. Figure 9 Shown are the ultraviolet-visible light absor...
Embodiment 3
[0061] Example 3: Fluorescence changes of NP-1 and NP-2 before and after white light illumination
[0062] Using white LED (62.6mW / cm 2 ) light illuminates the nanoparticle assembly obtained above, and performs a fluorescence emission spectrum test after 12 minutes of illumination. The test results show that: with the prolongation of the illumination time, the fluorescence at 825nm in the fluorescence emission spectrum increases, and the fluorescence at 760nm becomes weaker (excitation wavelength is 633nm), so it can indirectly reflect the release of nitric oxide. The test results are shown in Figure 11 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com