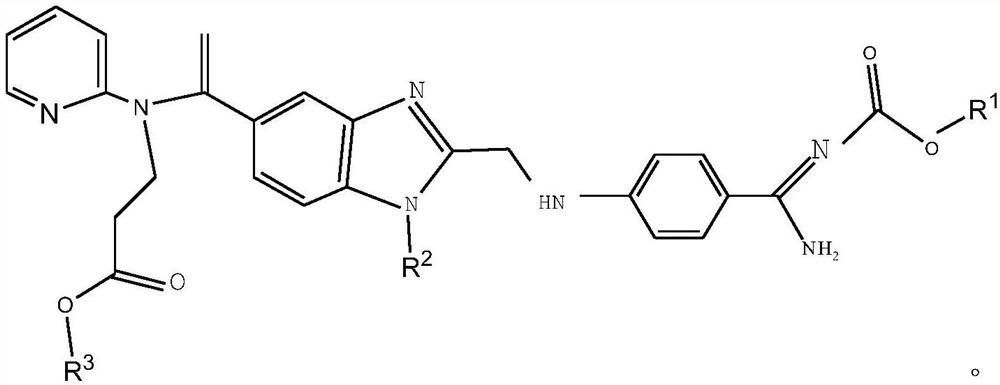
Synthesis method of dabigatran etexilate
A technology for dabigatran etexilate and a synthesis method is applied in the field of synthesis of dabigatran etexilate, can solve problems such as unfavorable large-scale industrial production, complicated and complicated synthesis steps, unfavorable industrialized production, etc. The effect of high purity and low amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
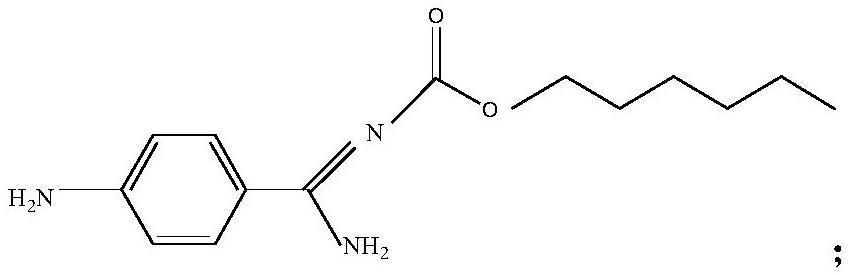
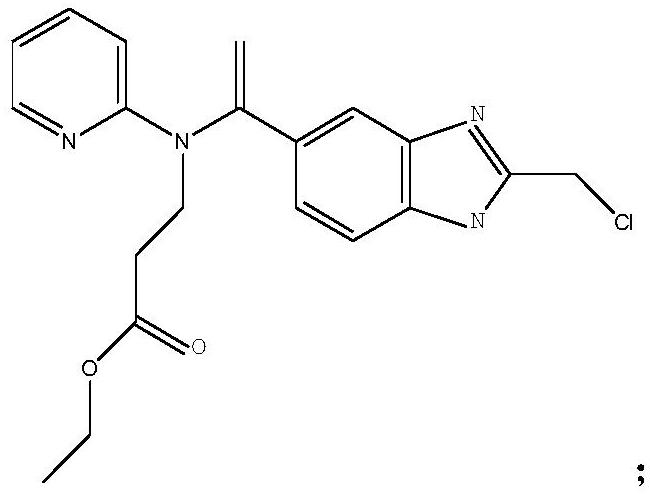
[0034] Add 350mL of ethyl acetate and 40.2g of (Z)-hexyl (amino(4-aminophenyl)methylene) carbamate successively in a 1L reaction flask, add dropwise 280g of 30% aqueous sodium hydroxide solution, at 45°C Heat to dissolve completely. Separate the layers, and wash the organic phase with water. To the combined organic phases were added 8.1 g sodium iodide, 20.4 g sodium bicarbonate, 5.2 g tetrabutylammonium bromide, 44.3 g 3-(1-(2-(chloromethyl)-1H-indole-5 -yl)vinyl)(phenyl)amino)ethyl propionate and 160mL of water were reacted at 70°C for 3h. Separate the liquid, wash the organic phase with water, cool down to 15°C, stir for 3.5h to precipitate a solid, and centrifuge. Recrystallize from ethyl acetate with 3 times the volume of crude dabigatran etexilate. Vacuum drying at 45° C. and 0.07 MPa for 8 hours gave 80.5 g of dabigatran etexilate with a yield of 96.9% and an HPLC purity of 99.85%.
Embodiment 2
[0036] Add 352mL of dichloromethane and 40.5g of (Z)-hexyl (amino(4-aminophenyl)methylene) carbamate successively in a 1L reaction flask, add dropwise 250g of 30% aqueous sodium hydroxide solution at 40°C Heat to dissolve completely. Separate the layers, and wash the organic phase with water. To the combined organic phases were added 6.0 g sodium iodide, 18.3 g sodium bicarbonate, 4.0 g tetrabutylammonium bromide, 41.2 g 3-(1-(2-(chloromethyl)-1H-indole-5 -yl)vinyl)(phenyl)amino)ethyl propionate and 160mL of water were reacted at 65°C for 2h. Separate the liquid, wash the organic phase with water, cool down to 10°C, stir for 3 hours to precipitate a solid, and centrifuge. Recrystallize with 2 times the volume of crude dabigatran etexilate in dichloromethane. Vacuum drying at 40°C and -0.06MPa for 7h gave 79.8g of dabigatran etexilate with a yield of 96.1% and a HPLC purity of 99.80%.
Embodiment 3
[0038] Add 440mL tetrahydrofuran and 40.3g (Z)-hexyl (amino(4-aminophenyl)methylene) carbamate successively to a 1L reaction flask, add dropwise 340g of 30% sodium hydroxide aqueous solution, and heat completely at 50°C dissolve. Separate the layers, and wash the organic phase with water. To the combined organic phases were added 12.3 g sodium iodide, 27.4 g sodium bicarbonate, 6.0 g tetrabutylammonium bromide, 52.4 g 3-(1-(2-(chloromethyl)-1H-indole-5 -yl)vinyl)(phenyl)amino)ethyl propionate and 200mL water were reacted at 75°C for 4h. Separate the liquid, wash the organic phase with water, cool down to 20°C, stir for 4 hours to precipitate a solid, and centrifuge. Recrystallize with 5 times the volume of crude dabigatran etexilate in tetrahydrofuran. Vacuum drying at 50° C. and 0.08 MPa for 8 hours gave 80.3 g of dabigatran etexilate with a yield of 96.3% and an HPLC purity of 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com