Lithium battery electrolyte additive and preparation method thereof
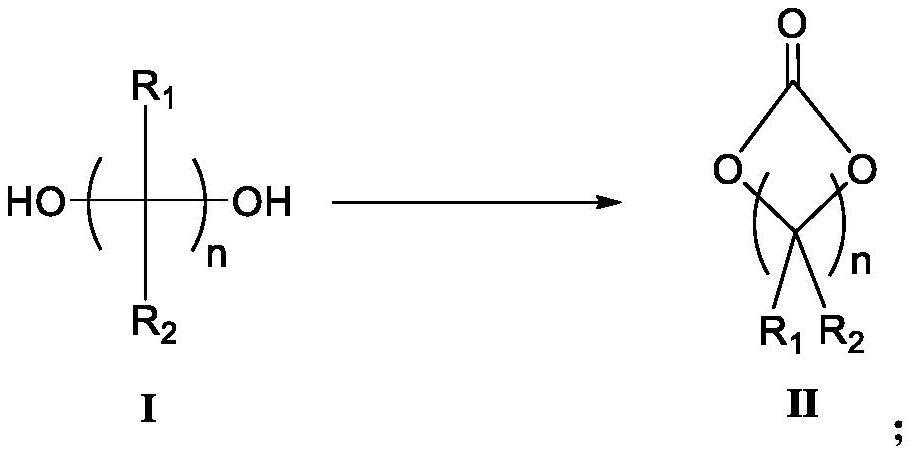
An electrolyte additive and lithium battery technology, which is applied in secondary batteries, circuits, electrical components, etc., can solve problems such as complex reaction and purification process, difficult product quality control, and highly toxic phosphorus trichloride. Green friendly, excellent solubility, good effect of high rate discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
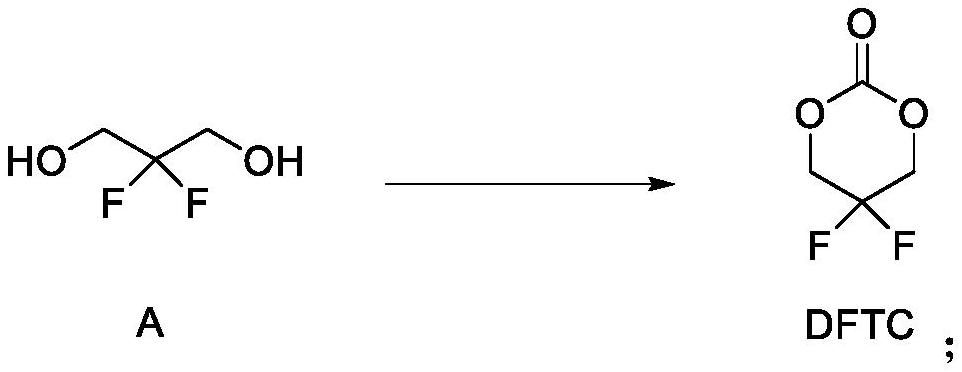
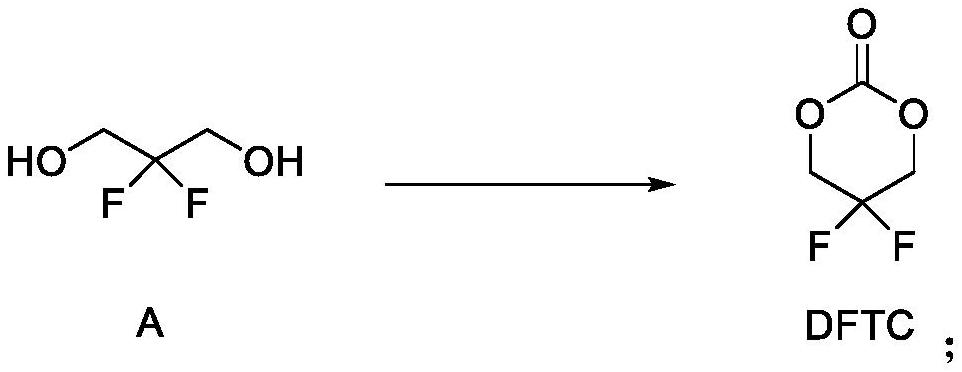
[0032] Preparation of DFTC: Under the protection of nitrogen, add 1 L of dichloromethane, 10 g of compound A (2,2-difluoro-1,3-propanediol), 89.2 mmol, into a dry and anhydrous 2 L reaction flask, and add them in batches under stirring at room temperature CDI 15.92g, 98.1mmol, the mixture was heated to reflux at 40°C and kept to react for 4h, confirmed by TLC that the reaction was complete, cooled to 5°C, added 400ml of 2mol / L HCl aqueous solution to the system to quench the reaction, separated the liquid, and used dichloro Extracted with methane to no product (2 times × 200ml), the combined organic phases were washed once with water × 300ml, washed once with saturated sodium chloride solution × 300ml, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure (vacuum degree -0.098 MPa) to solvent-free to obtain the crude product, and obtain pure DFTC 9.66g as a colorless liquid by distillation under reduced ...
Embodiment 2
[0038]
[0039] Preparation of DFTC: Under the protection of nitrogen, add 100ml of dichloromethane, 5g of compound A (2,2-difluoro-1,3-propanediol), 44.6mmol, into a dry and anhydrous 250ml reaction flask, add in batches under stirring at room temperature Triphosgene 14.8g, 49.1mmol, the mixture was heated to reflux at 40°C and incubated for 4h, confirmed by TLC that the reaction was complete, cooled to 5°C, added 40ml of water to the system to quench the reaction, separated, and the aqueous phase was extracted with dichloromethane to No product (2 times × 30ml), the combined organic phases were washed once with water × 30ml, washed once with saturated sodium chloride solution × 30ml, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure (vacuum degree -0.098MPa) to The crude product was obtained after solvent-free, and 3.4 g of pure DFTC was obtained as a colorless liquid by distillation under reduced pressure....
Embodiment 3
[0045]
[0046]Preparation of DFTC: Under the protection of nitrogen, add 100L of dichloromethane, 5.0kg of compound A (2,2-difluoro-1,3-propanediol), 44.6mol, into a dry and anhydrous 200L reactor, and stir in batches at room temperature Add 7.96kg, 49.1mol of CDI, heat the mixture to reflux at 40°C and keep it warm for 4 hours, confirm the completion of the reaction by TLC, cool down to 5°C, add 2mol / L H to the system 2 SO 4 Quench the reaction with 40L of solution, separate the liquids, extract the aqueous phase with dichloromethane until there is no product (2 times × 20L), wash the combined organic phase with water once × 30L, wash once with saturated sodium chloride solution × 30L, anhydrous sodium sulfate After drying and suction filtration, the filtrate was concentrated under reduced pressure (vacuum degree-0.098MPa) to obtain a crude product after being free of solvent, and 5.175kg of pure DFTC was obtained as a colorless liquid by vacuum distillation.
[0047] Yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com