Preparation process of fibrinogen
A fibrinogen and preparation process technology, applied in the field of blood products, can solve the problems of increasing process time and equipment cost, the influence of lifespan on chromatographic effects, and increasing process risks, so as to ensure purity and recovery rate, shorten process time, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
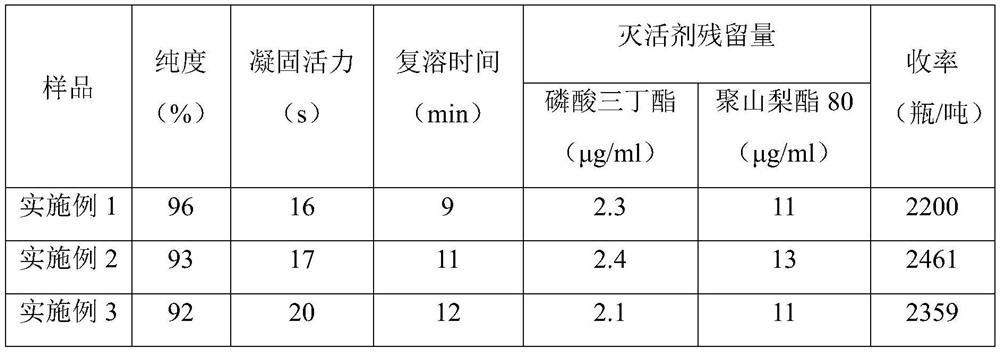
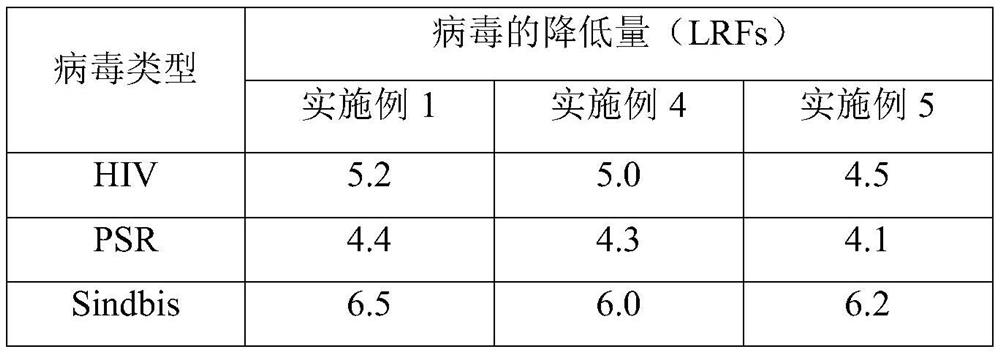
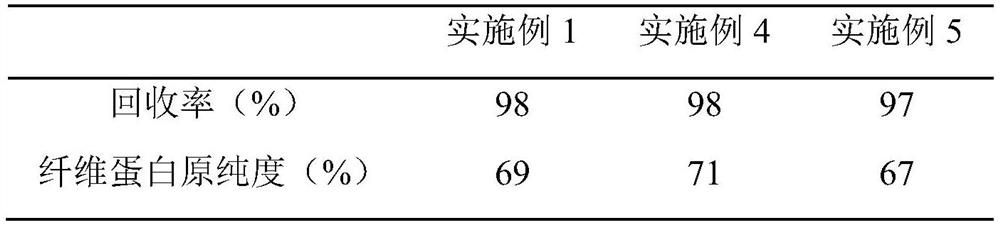
Embodiment 1
[0040] The preparation process 1 of embodiment 1 fibrinogen
[0041] (1) Preparation of component I: add A-50 gel with 2.0% plasma weight to the cryoprecipitate supernatant, stir and absorb for 60 minutes, let stand for more than 40 minutes to separate, and collect the eluate for prothrombin complex production; collect the flow Make component I through the liquid; add 15°C, 0.9% sodium chloride solution to the flow-through liquid, adjust the pH value of the flow-through liquid to 7.15 with pH4.0 acetate buffer, add -15°C at 0°C, 95% ethanol to a final concentration of 8% v / v, control the temperature at -2.5°C, continue stirring for 2 hours, control the temperature at -3°C, feed the liquid at 50L / min, rotate at 4800 rpm, and centrifuge to obtain Component I precipitates.
[0042] (2) The first step of impurity removal: Precipitate the component I prepared in step (1), chop it up, add 5 times the volume of dissolving solution A to dissolve, stir for 1 hour to prevent foam gener...
Embodiment 2
[0050] The preparation process 2 of embodiment 2 fibrinogen
[0051] (1) Preparation of component I: add A-50 gel with 3.0% plasma weight to the cryoprecipitate supernatant, stir and absorb for 60 minutes, let stand for more than 40 minutes for separation, and collect the eluate for prothrombin complex production; collect the flow Make component I through the liquid; add 0°C, 0.9% sodium chloride solution to the flow-through liquid, adjust the pH value of the flow-through liquid to 7.20 with pH4.0 acetate buffer, add -20°C at 0°C, 95% ethanol to a final concentration of 8% v / v, control the temperature at -2.0°C, continue stirring for 2 hours, control the temperature at -3°C, feed the liquid at 50L / min, rotate at 5200 rpm, and centrifuge to obtain Component I precipitates.
[0052] (2) The first step of impurity removal: Precipitate the component I prepared in step (4), chop it up, put it into 8 times solution A to dissolve, stir to dissolve, stir for 1.5h, prevent foam genera...
Embodiment 3
[0060] The preparation process 3 of embodiment 3 fibrinogen
[0061] (1) Preparation of component I: add A-50 gel with 4.5% plasma weight to the cryoprecipitate supernatant, stir and adsorb for 60 minutes, let stand for more than 40 minutes to separate, and collect the eluate to make prothrombin complex; collect the flow Make component I through the solution; add 10°C, 0.9% sodium chloride solution to the flow-through solution, adjust the pH value of the flow-through solution to 7.30 with pH4.0 acetate buffer, add -10°C at 0°C, 95% ethanol to a final concentration of 8% v / v, control the temperature at -2.5°C, continue to stir for 2 hours, control the temperature at 0°C, feed the liquid at 50L / min, rotate at 5000 rpm, and centrifuge to obtain the composition Part I precipitation.
[0062] (2) The first step of impurity removal: Precipitation of component I prepared in step (1), chopping, adding solution A of 7 times the weight of precipitation, stirring to dissolve, stirring f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com